Abstract
Introduction
Guidelines recommend screening older people (> 60–65 years) with type 2 diabetes (T2D) for cognitive impairment, as it has implications in the management of diabetes. The Montreal Cognitive Assessment (MoCA) is a sensitive test for the detection of mild cognitive impairment (MCI) in the general population, but its validity in T2D has not been established.
Methods
We administered MoCA to patients with T2D (age ≥ 60 years) and controls (no T2D), along with a culturally validated neuropsychological battery and functional activity questionnaire. MCI was defined as performance in one or more cognitive domains ≥ 1.0 SD below the control group (on two tests representing a cognitive domain), with preserved functional activities. The discriminant validity of MoCA for the diagnosis of MCI at different cut-offs was ascertained.
Results
We enrolled 267 patients with T2D and 120 controls; 39% of the participants with T2D met the diagnostic criteria for MCI on detailed neuropsychological testing. At the recommended cut-off on MoCA (< 26), the sensitivity (94.2%) was high, but the specificity was quite low (29.5%). The cut-off score of < 23 showed an optimal trade-off between sensitivity (69.2%), specificity (71.8%), and diagnostic accuracy (70.8%). The cut-off of < 21 exhibited the highest diagnostic accuracy (74.9%) with an excellent specificity (91.4%), a good positive and negative predictive value (78.5% and 73.7%, respectively).
Conclusions
The recommended screening cut-off point on MoCA of < 26 has a suboptimal specificity and may increase the referral burden in memory clinics. A lower cut-off of < 21 on MoCA maximizes the diagnostic accuracy.
Interactive Visual Abstract available for this article.
Plain Language Summary
Type 2 diabetes (T2D) is a risk factor for cognitive dysfunction which potentially impacts diabetes self-management skills. Guidelines recommend screening older adults with diabetes for early detection of cognitive impairment. For screening cognitive impairment in busy endocrine clinics, we need a test that is easy and rapid to administer, sensitive enough to pick the cognitive deficits of T2D and at the same time gives less false-positive outcomes. The Montreal Cognitive Assessment (MoCA) scale is a widely available cognitive screening tool, but there are no studies evaluating its discriminant properties in people with diabetes. We evaluated the performance metrics of MoCA in this population. We found mild cognitive impairment in four out of ten participants with T2D at or above 60 years of age. At the recommended cut-off on MoCA (< 26), the sensitivity was high, but the specificity quite low. We found better diagnostic accuracy at lower cut-offs (20/21), with high specificity but a lower sensitivity. At this cut-off, approximately one out of five people screened using MoCA would require detailed neuropsychological testing, and four out of five who undergo detailed evaluation would have true cognitive impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cognitive impairment in diabetes has important implications in management, hence guidelines recommend cognitive screening of older adults with diabetes. |
Montreal Cognitive Assessment (MoCA) is a globally used cognitive screening test that is quick to administer, widely available, and sensitive to pick executive dysfunction (most prominent cognitive deficit in people with diabetes). |
Using actuarial criteria to define mild cognitive impairment (MCI), we found MCI in 39% participants with type 2 diabetes at or above 60 years of age (dysexecutive type was the most frequent). |
At the recommended cut-off on MoCA (< 26), the sensitivity (94.2%) was high, but the specificity quite low (29.5%), implicating a higher referral burden for detailed neuropsychological testing. |
A lower cut-off, with a better (20/21) diagnostic accuracy (74.9%), may be a pragmatic answer to cognitive testing in busy endocrine clinics (specificity 91.4%, sensitivity of 49.0%, positive predictive value 78.5%, negative predictive value of 73.7%). |
Interactive Visual Abstract
This article includes an interactive visual abstract to facilitate understanding of the article. Please see a preview below. To view the interactive version, click here: https://cloudstore.springerhealthcare.com/adis/infographics/gupta-a-et-al-interactive-infographic-diabetes-in-therapy/adis__iVA_17005180400_final.html

Introduction
Type 2 diabetes (T2D) is a risk factor for cognitive dysfunction and is associated with a 43% increased risk for dementia and a 49% increased risk for mild cognitive impairment (MCI) compared to people without diabetes [1]. It is associated with a 53% increased conversion rate of MCI to dementia and when poorly controlled it triples the risk of progression [2, 3]. The prevalence of diabetes is increasing worldwide. As per the 10th atlas of International Diabetes Federation, 537 million adults (20–79 years) have diabetes [4]. The numbers are expected to increase to 643 million by 2030. Similarly, the current burden of dementia stands at 55 million, and nearly 10 million people are added to this figure every year [5]. With an ageing population and increasing diabetes prevalence, the burden of dementia is expected to increase enormously.
Cognitive impairment impacts diabetes self-management skills [6]. It is associated with increased hospital admissions, severe hypoglycemic episodes, major cardiovascular events, and death [6]. Early detection of cognitive impairment in T2D is important to minimize the risks involved with intensive diabetes management, to devise strategies to assist the patient in his diabetes self-management routine, and to reduce the risk of progression to dementia [7]. With upcoming disease-modifying therapies for some types of dementia [recently Food and Drug administration (FDA) approved anti-amyloid therapies for Alzheimer’s disease] [8], the stance on screening for cognitive impairment in T2D may gain a strong foothold in the future. The American Diabetes Association (ADA) recommends annual screening of older adults with diabetes (65 years or older) for early detection of MCI or dementia using tools like Mini-Mental State Examination, Mini-Cog, and the Montreal Cognitive Assessment (MoCA) [9]. ADA also recommends that screening be considered when the patient presents with increased problems with self-care activities, such as errors in calculating insulin doses and difficulty recognizing, preventing, or treating hypoglycemia. The Endocrine Society suggests screening of cognitive impairment every 2–3 years if the initial screening is normal, otherwise every year [6]. For screening cognitive impairment in endocrine clinics, we need a test that is easy and rapid to administer, sensitive enough to pick the cognitive deficits of T2D, and at the same time have less false-positive outcomes. MoCA is available in nearly 100 languages, and takes approximately 10 min to administer [10, 11].
However, the issue with MoCA is that the original cut-off of 26 proposed by Nasreddine et al. has been found to diagnose many false-positive MCIs, thereby more referrals to the specialized memory/dementia clinics for detailed neuropsychological testing. Further adjustment of one point to correct the influence of education for individuals with 12 or fewer years of formal education is not seen to give reliable results [12]. A recent meta-analysis has shown that the ideal cut-off for a diagnosis of MCI could be 23 [12]. However, there are no studies evaluating the discriminant performance of MoCA at various cut-offs, specifically in patients with diabetes [13], who have higher chances and a relatively different spectrum of cognitive deficits. The lack of data from South Asia is another lacuna in the literature.
The aim of this study was to evaluate the performance metrics of MoCA to detect cognitive dysfunction in people with T2D aged 60 years and above. We used a culturally adapted Indian Council of Medical Research (ICMR) neuropsychological battery for detailed neuropsychological testing [14]. We feel that such work is essential from India (South Asia), as the region has a substantial burden of diabetes, and with an ageing population will see enormous numbers with cognitive impairment.
Methods
Settings and Study Design
This cross-sectional study enrolled elderly participants (age ≥ 60 years) between 2020 and 2022 at the All India Institute of Medical Sciences, a tertiary care public hospital in New Delhi (North India). The institute's ethics committee approved the study (Ref. No. IEC-485/02.08.2019, AA-6/04.10.2019), and participants gave written informed consent. We performed the study according to the ethical standards of the Declaration of Helsinki and its subsequent amendments.
Objectives
The primary objective was to evaluate the test characteristics (sensitivity, specificity, positive and negative likelihood ratios, positive and negative predictive values, and diagnostic accuracy) of MoCA as a screening test for cognitive impairment and validate against a detailed regional neuropsychological battery in people with T2D.
Inclusion and Exclusion Criteria
We included participants with T2D (age ≥ 60 years) who were followed up at the Neurology and Endocrinology outpatient departments of our institute. We excluded those who were unwilling to participate, were known cases of major depressive disorder or psychosis, those with overt hypothyroidism [thyroid stimulating hormone (TSH) > 10 with subnormal T4], established dementia, disabling stroke, active delirium, significant visual, language or hearing disability interfering with neuropsychological testing, and impaired instrumental activities of daily living (cognitive disability index score ≥ 16, on the Scale for the Instrumental Activity of Daily Living in the Elderly (IADL-EDR) [15]). We also included a control group (age ≥ 60 years, without T2D) for comparison and for deriving the cut-offs to define cognitive impairment. These participants were either people seeking care (enrolled from the same clinics which provided cases) or were spouses of the cases. For the control group, we used the same exclusion criteria as cases, with the additional exclusion of those with malignancy, history of any stroke, Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) score ≥ 3.27. IQCODE is a structured questionnaire that captures the history of cognitive decline over the last 10 years as elicited from the informant, and a score ≥ 3.27 indicates a possible presence of dementia [16, 17].
Measurements
Clinical Interview
All participants underwent a clinical interview to obtain information on sociodemographic parameters, medical history (duration of diabetes, history of hypertension, coronary artery disease, stroke, transient ischemic attack, smoking, alcohol consumption, details on medications, diabetes-related micro and macrovascular complications), depression [Patient health Questionnaire-9 (PHQ-9)] followed by anthropometric and biochemical measurements [18].
Psychological Testing
The Montreal Cognitive Assessment Scale (MoCA) We used the Hindi or English version of the MoCA. It assesses the following cognitive domains: orientation (time), visuospatial (cube copying, clock drawing), naming (three-line drawings of animals), language (sentence repetition and verbal fluency), memory (delayed recall of a five-word list) and attention/executive [forward (five digits) and backward (three digits) digit span, tapping to the letter 'A', serial subtraction (100–7), alternating trail making (five-point spread of alternating numbers and alphabets), abstraction (similarity between items), phonemic fluency ka] functions. It takes 10 min to administer. The maximum score is 30. If the patient has 12 or fewer years of education, one point is added to his final score [10].
Neuropsychological Battery The detailed neuropsychological battery (Indian Council of Medical Research Neurocognitive toolbox or ICMR-NCTB) included the following tests—Trail Making A & B, Phonemic fluency ka, ma, pa (Attention/Working memory/Executive functions); Verbal learning test—total learning, learning over trials, delayed recall, delayed recognition (verbal memory); Modified Taylor Complex Figure (MTCF) test—immediate and delayed recall (visual memory); MTCF copy and line bisection test (Visuo-perceptual functions); and Picture naming test, Category fluency for animals, vegetables, food (language) [14]. It is a culturally validated tool. We used a total of 17 tests under five cognitive domains. The IQCODE and IADL-EDR are also part of the toolbox. Certified psychologists administered the above battery, including MoCA. We obtained written permission to use ICMR-NCTB from Indian Council of Medical Research.
Definition of Mild Cognitive Impairment
MCI was defined using actuarial criteria based on neuropsychological testing [19]. As per the actuarial criteria, impairment in a cognitive domain was defined as performance ≥ 1.0 SD below the control group on at least two tests representing a cognitive domain under evaluation. To differentiate MCI from mild dementia, instrumental activities of daily living (iADLs) were supposed to be normal in MCI cases (all participants had normal iADLs, as impairment in the same was an exclusion criteria).
Test Characteristics of MoCA for MCI, with ICMR-NCTB as the Reference Standard
The sensitivity, specificity, positive and negative likelihood ratios, positive predictive value (PPV), negative predictive value (NPV) and percent correctly diagnosed, were calculated to determine the discriminant validity of MoCA for detecting cognitive impairment (on neuropsychological testing) using an online calculator from MedCalc. We evaluated the original cut-off of 26 proposed by Nasreddine et al. and a lower cut-off (cut-off 23) proposed by a recent meta-analysis [10, 12]. We further evaluated the test characteristics at other cut-offs to provide the readers and researchers with the desired information for their interpretation for clinical or research purpose.
Statistical Analysis
We analyzed data using Stata 15.0 (StataCorp, College Station, TX, USA) and presented data as number (%), mean ± SD, or median (Q25–Q75) as appropriate. Pearson chi-square test or Fisher's exact test were used (as appropriate) for qualitative variables. For quantitative variables, normality was assessed using the Shapiro–Wilk test. Student's t test for independent samples was used to compare the difference in means for the normally distributed quantitative variables. Wilcoxon rank-sum test was used for quantitative variables without normal distribution. A p value of < 0.05 was taken significant.
For each of the 17 psychological tests, a z-score was calculated [z-score = (individual participant raw score—control group mean)/control group SD] for each participant. The mean and standard deviation were derived by pooling data from all the controls. For all the tests, a higher z-score indicated a better performance except for trail making test (TMT) —A & B. The signs of these two tests were inverted for a uniform interpretation. For a given test, impairment was defined as a z-score ≥ 1.0. For a given domain, impairment was defined as any two tests (representing that domain) with z-scores ≥ 1.0. The frequency of overall MCI was calculated as the number of participants with cognitive impairment in any of the five domains [performance ≥ 1.0 SD below the control group (z-scores ≥ 1.0) on at least two tests of any one cognitive domain]. The test characteristics of MoCA for MCI using the ICMR detailed neuropsychological battery as reference were evaluated using online calculator from MedCalc.
Results
Baseline Characteristics
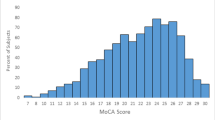
We recruited 267 participants (male 68.5%) with T2D and 120 (male 50.6%) without T2D (controls). Among participants with T2D, the mean ± SD age was 65.2 ± 4.2 years, and mean duration of formal education was 12.2 ± 4.4 years (Table 1). The median (interquartile range: IQR) duration of diabetes was 10 (5, 18) years, with a mean HbA1c of 7.9 ± 1.8%. Hypertension was present in 183 (68.5%), and 162 (60.9%) were overweight or obese. Coronary artery disease and cerebrovascular disease (history of non-disabling stroke) were present in 54 (20.2%) and 14 (5.2%), respectively. The control group had a significantly lower frequency of men (50.6 vs. 68.5%; p < 0.001), employed participants (16.7 vs. 24.7%; p = 0.004), and years of formal education (10.9 ± 4.6 vs. 12.2 ± 4.4; p = 0.009) compared to cases. The details on baseline characteristics are presented in Table 1. The raw scores of the psychological tests for cases and controls are presented in Table 2.
Frequency of MCI Based on Neuropsychological Assessment
The overall frequency of MCI in participants with T2D was 39.0% (104/267). The most frequently affected domain was the attentional network (26.2%; n = 70), followed by memory involvement (verbal: 14.6% {n = 39}, visual: 12.4% {n = 33}). Language was affected in 9.7% (n = 26) and visuo-perceptual functions in 2.3% (n = 6). More than one domain was affected in 18.4% (n = 49) (Table 3).
Discriminant Validity of MoCA
The original cut-off score of 25/26 (cut-off 25/26 indicates < 26 is abnormal) had a high sensitivity (94.2%), but very low specificity (29.5%) and accuracy (54.7%) (Table 4). The cut-off of 22/23, exhibited an optimal trade-off between sensitivity, specificity, and diagnostic accuracy (sensitivity 69.2%, specificity 71.8%, NPV 78.5%, PPV 61.1%, accuracy 70.8%). The cut-off point of 24/25 was the lowest cut-off with > 80% sensitivity and NPV (sensitivity 89.4%, specificity 49.7%, NPV 88.0%, PPV 53.2%, accuracy 65.2%). We found the maximum accuracy of 74.9% at the cut-off point of 20/21. The other test characteristics at this cut-off point included sensitivity 49.0%, specificity 91.4%, PPV 78.5%, and NPV of 73.7%.
Implications of Different Cut-Offs
Using the cut-off 22/23, 44.2% patients would need a referral to a memory clinic, out of which (referred cases) 61% would be diagnosed to have cognitive impairment on detailed neuropsychological tests. It would pick up 69.2% of the actually cognitively impaired out of the whole cohort. The cut-off of 25/26 would pick up 94.2% of the cognitively impaired participants in the cohort. However, it would need 79.7% referrals to the memory clinic, and only 46% of those referred would turn out to have cognitive impairment on detailed tests. At the cut-off 20/21, 19.1% patients would need referral to a memory clinic, and 78.5% of referred cases would turn out positive on detailed tests. It would pick up 49% of the cognitively impaired in the group (Table 5).
Discussion
In this study, 39.0% of the participants with T2D met the diagnostic criteria for MCI on detailed neuropsychological testing. As the attentional networks were most frequently affected, the dysexecutive type of MCI was the most prevalent. The cut-off score of 22/23 (score < 23 abnormal) showed an optimal trade-off between sensitivity (69.2%), specificity (71.8%), and diagnostic accuracy (70.8%), while the cut-off of 20/21 exhibited the highest diagnostic accuracy (74.9%) with specificity 91.4%, sensitivity 49.0%, PPV 78.5%, and NPV of 73.7%.
The current study found MCI in 39.0% of elderly with T2D. We used actuarial criteria based on detailed neuropsychological testing to define MCI. A recent systematic review and meta-analysis of 12 studies (3906 subjects with diabetes, age range 33–81 years) found that the overall prevalence of MCI in T2D was 45% (95% CI 36, 54) [20]. MoCA (cut-off 26) was used as the lone criteria in three studies, and the reported prevalence at this cut-off was variable 37.9, 58.6, and 67.3%.
Although, the most commonly used cut-off of MoCA for MCI in the general population is 26 (score < 26 abnormal), there are studies which have found that this widely recommended cut-off leads to an inflated rate of false positives, (particularly in the elderly and people with lower education), and the cut-off score of 23 yields the best diagnostic accuracy [12]. A recently published systematic review evaluating the accuracy of MoCA for detecting mild cognitive impairment in the general population (13 studies, 2158 participants, 44% with MCI, mean age 72 years, mean education 13.5 years, race 93.4% Caucasian) also supports a lower cut-off [21]. In this meta-analysis, sensitivity and specificity were 73.5% and 91.3% respectively at cut-off < 23, while 93.7% and 58.8%, respectively, at cut-off < 26. In our study, sensitivity and specificity were 69.2% and 71.8% at cut-off < 23. At cut-off < 26, the sensitivity (94.2%) was high, but the specificity (29.5%) quite low.
There are meagre data in literature on the discriminant properties of screening tools, particularly MOCA, for detecting cognitive impairment in people with T2D. As per a systematic review (on the utility of brief cognitive tests for assessing cognitive decrements in patients with T2D) 22 studies used a brief cognitive test, either for screening purpose or as a measure of global function in this population [13]. Out of these, only one study reported discriminant functions (sensitivity, specificity, positive predictive value, negative predictive value) of the brief tool that was used against a detailed neuropsychological evaluation [22]. The brief tool used here was MMSE, which has a limited sensitivity to diagnose MCI and executive dysfunction (most frequent cognitive deficit found in T2D) and may have ceiling effects. From this perspective, our study is a valuable addition to literature as it is the first one to study the psychometric properties of MoCA for detection of cognitive dysfunction in T2D, with detailed neuropsychological tests as the reference.
This study has important clinical and research implications. Considering the facts that in addition to potential benefits, screening for cognitive impairment in T2D has its own pitfalls and counterarguments (in the form of lack of specific therapy for cognitive impairment, slow progression, costs, and distress involved in follow-up in terms of finances, time, and health personnel involved), for clinical practice, one needs to choose a test with high accuracy. In our study, the cut-off of 20/21 showed an excellent specificity (91.4%) and positive predictive value (78.5%) with a good diagnostic accuracy (74.9%). In the clinic, patients with scores ≥ 21 on screening, may not require detailed neuropsychological assessment until they report cognitive concerns, or impairment in functional activities or problems with diabetes self-management, and may be followed up annually. Patients scoring below this cut-off could be referred to a memory clinic to decide if they need a detailed neuropsychological work up. Using MoCA alone to define MCI (particularly with the recommended cut-off of 26) may not identify the correct target population.
Strengths and limitations This is the first study to assess the psychometric properties of MoCA for detection of cognitive dysfunction in elders with T2D, at different cut-offs. We used a culturally validated detailed neuropsychological battery as reference for diagnosis of MCI. With limited data from low- and middle-income countries, the information from our study could be helpful in building evidence for cognitive screening in people with T2D, especially in South Asia, which caters a huge chunk of the global burden of diabetes. The limitations include a lack of information on generalizability of findings to other ethnicities and middle-aged individuals with T2D. The control group had fewer years of education (1.3 years), and a lower frequency of men and employed participants. Since the cognitive scores of the control group were used to derive the patient z-scores, this could have underestimated the frequency of cognitive impairment in patients with T2D in our study.
It is also crucial to mention that the diabetes population is not a homogenous group. Multiple variables like age, education, presence or absence of depression, duration of diabetes, additional burden of cardiovascular risk factors, diabetes-related vascular complications, hypoglycemia (severity, frequency, duration of episodes) and drugs could influence the performance of MoCA in persons living with diabetes [23, 24]. In addition, it has been observed that newer diabetes drugs like glucagon-like peptide-1 agonists, sodium-glucose co-transporter 2, and dipeptidyl peptidase-4 inhibitors could positively influence cognition [25, 26]. Though we feel that sample size of this study was sufficient to evaluate the utility of MoCA in diabetes, any sub-group analysis (taking above factors into account) would ideally require a well-established large prospective cohort of newly diagnosed diabetes, with careful documentation of all associated factors.
To conclude, our study found the highest accuracy at lower cut-off of MoCA for cognitive impairment in the Indian population with T2D aged 60 years and above. Similar studies from other ethnicities could strengthen the screening protocol of cognitive dysfunction in T2D.
Data Availability
The datasets generated during and/or analyzed during the current study will be available from the corresponding author on reasonable request.
References
Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. 2019;55: 100944.
Pal K, Mukadam N, Petersen I, Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2018;53(11):1149–60.
Dove A, Shang Y, Xu W, Grande G, Laukka EJ, Fratiglioni L, et al. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimers Dement. 2021;17(11):1769–78.
IDF Diabetes atlas 2021. Available from https://diabetesatlas.org/atlas/tenth-edition/. Accessed 12 June 2023.
Dementia. Available from https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed 12 June 2023.
LeRoith D, Biessels GJ, Braithwaite SS, Casanueva FF, Draznin B, Halter JB, et al. Treatment of diabetes in older adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1520–74.
Biessels GJ, Whitmer RA. Cognitive dysfunction in diabetes: how to implement emerging guidelines. Diabetologia. 2020;63(1):3–9.
Cummings J. Anti-amyloid monoclonal antibodies are transformative treatments that redefine Alzheimer’s disease therapeutics. Drugs. 2023;83(7):569–76.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, on behalf of the American Diabetes Association, et al. 13. Older Adults: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(1):S216–29.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
MoCA cognition. Available from https://mocacognition.com. Accessed 12 June 2023.
Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33(2):379–88.
Dong Y, Kua ZJ, Khoo EY, Koo EH, Merchant RA. The utility of brief cognitive tests for patients with type 2 diabetes mellitus: a systematic review. J Am Med Dir Assoc. 2016;17(10):889–95.
Menon RN, Varghese F, Paplikar A, Mekala S, Alladi S, Sharma M, et al. Validation of Indian Council of Medical Research neurocognitive tool box in diagnosis of mild cognitive impairment in India: lessons from a harmonization process in a linguistically diverse society. Dement Geriatr Cogn Disord. 2020;49(4):355–64.
Mathuranath PS, George A, Cherian PJ, Mathew R, Sarma PS. Instrumental activities of daily living scale for dementia screening in elderly people. Int Psychogeriatr. 2005;17(3):461–74.
Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med. 1991;21(3):785–90.
Informant questionnaire on cognitive decline in the elderly. Available from https://nceph.anu.edu.au/research/tools-resources/informant-questionnaire-cognitive-decline. Accessed 12 June 2023.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42(1):275–89.
You Y, Liu Z, Chen Y, Xu Y, Qin J, Guo S, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. 2021;58(6):671–85.
Islam N, Hashem R, Gad M, Brown A, Levis B, Renoux C, Thombs BD, McInnes MD. Accuracy of the Montreal Cognitive Assessment tool for detecting mild cognitive impairment: a systematic review and meta-analysis. Alzheimers Dement. 2023;19(7):3235–43. https://doi.org/10.1002/alz.13040.
Koekkoek PS, Rutten GE, van den Berg E, van Sonsbeek S, Gorter KJ, Kappelle LJ, et al. The “Test Your Memory” test performs better than the MMSE in a population without known cognitive dysfunction. J Neurol Sci. 2013;328(1–2):92–7.
Savelieff MG, Chen KS, Elzinga SE, Feldman EL. Diabetes and dementia: clinical perspective, innovation, knowledge gaps. J Diabetes Complicat. 2022;36(11):108333.
van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(4):325–36.
Tang H, Shao H, Shaaban CE, Yang K, Brown J, Anton S, et al. Newer glucose-lowering drugs and risk of dementia: a systematic review and meta-analysis of observational studies. J Am Geriatr Soc. 2023;71:2096–106.
Moran C, Callisaya ML, Srikanth V, Arvanitakis Z. Diabetes therapies for dementia. Curr Neurol Neurosci Rep. 2019;19(8):58.
Acknowledgements
We thank the participants of the study.
Funding
This project has been funded by Indian Council of Medical Research (Grant No 5/4/5–4/Diab/19-NCD-II). Anu Gupta is the primary recipient of this grant. The sponsor has no role in study design, data collection, analysis and interpretation, writing of the report and the decision to submit the report for publication. No funding or sponsorship was received for publication of this article.
Author information
Authors and Affiliations
Contributions
Anu Gupta and Yashdeep Gupta conceptualized the study, collected, and analyzed the data. Anu Gupta wrote the first draft. Anu Gupta, Yashdeep Gupta, Alpesh Goyal, Roopa Rajan, Venugopalan Y Vishnu, Mani Kalaivani, Nikhil Tandon, and Madakasira V.P. Srivastava critically read and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors (Anu Gupta, Yashdeep Gupta, Alpesh Goyal, Roopa Rajan, Venugopalan Y Vishnu, Mani Kalaivani, Nikhil Tandon, and Madakasira V.P. Srivastava) have nothing to disclose.
Ethical Approval
We performed this study at the All India Institute of Medical Sciences, New Delhi, India. The institute's ethics committee approved the study (Ref. No. IEC-485/02.08.2019, AA-6/04.10.2019), and participants gave written informed consent. We performed the study according to the ethical standards of the Declaration of Helsinki and its subsequent amendments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gupta, A., Goyal, A., Rajan, R. et al. Validity of Montreal Cognitive Assessment to Detect Cognitive Impairment in Individuals with Type 2 Diabetes. Diabetes Ther 15, 1155–1168 (2024). https://doi.org/10.1007/s13300-024-01549-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01549-y