Abstract
Application of continuous glucose monitoring (CGM) has moved diabetes care from a reactive to a proactive process, in which a person with diabetes can prevent episodes of hypoglycemia or hyperglycemia, rather than taking action only once low and high glucose are detected. Consequently, CGM devices are now seen as the standard of care for people with type 1 diabetes mellitus (T1DM). Evidence now supports the use of CGM in people with type 2 diabetes mellitus (T2DM) on any treatment regimen, not just for those on insulin therapy. Expanding the application of CGM to include all people with T1DM or T2DM can support effective intensification of therapies to reduce glucose exposure and lower the risk of complications and hospital admissions, which are associated with high healthcare costs. All of this can be achieved while minimizing the risk of hypoglycemia and improving quality of life for people with diabetes. Wider application of CGM can also bring considerable benefits for women with diabetes during pregnancy and their children, as well as providing support for acute care of hospital inpatients who experience the adverse effects of hyperglycemia following admission and surgical procedures, as a consequence of treatment-related insulin resistance or reduced insulin secretion. By tailoring the application of CGM for daily or intermittent use, depending on the patient profile and their needs, one can ensure the cost-effectiveness of CGM in each setting. In this article we discuss the evidence-based benefits of expanding the use of CGM technology to include all people with diabetes, along with a diverse population of people with non-diabetic glycemic dysregulation.
Similar content being viewed by others
Continuous glucose monitoring (CGM) devices are proven to improve glycemic management for people with type 1 diabetes or type 2 diabetes, as well as lowering the risk of acute diabetes complications and hospital admissions. |
CGM may be used on a day-to-day or intermittent basis, depending on the profile and needs of the person with diabetes, such that the most cost-effective use of CGM is achieved. |
Wider use of CGM should include people with cystic fibrosis-related diabetes and women with gestational diabetes mellitus, as well as in hospital settings where stress-induced hyperglycemia has clinical consequences for inpatients without diabetes. |
Use of CGM can be tailored to meet the needs of people with diabetes and also people with glycemic dysregulation for other reasons, including inpatients in acute care settings. |
Introduction
The landscape of treating and managing diabetes has been transformed over the past decade by the increased availability and application of continuous glucose monitoring (CGM) technology, which provides people with diabetes and their healthcare professionals (HCPs) with on-demand glucose information. This includes not only the current glucose reading for the user, at any time of day, but also important information on whether their glucose levels are stable, rising, or falling. The glucose data collected by CGM devices is also used to generate important patterns and trends that can be analyzed retrospectively to understand the overall glycemic balance of the user, and that can be used to guide therapeutic decisions on diabetes care. These capabilities go far beyond the limitations of traditional fingerprick self-monitoring of blood glucose (SMBG) testing, which is often painful for the user and provides only a one-time snapshot of glucose levels when a test is conducted. The use of CGM also enables parents and caregivers to monitor glucose levels in school-age children or elderly people remotely, using smartphone apps, which allows an additional level of care for people with diabetes as they participate in different activities.
Commercially available CGM devices provide a measurement of glucose levels in the interstitial fluid (ISF) in the subcutaneous space [1, 2], either using a thin sensor filament that is inserted into the subcutaneous space (transcutaneous) or by insertion of the sensor itself into the subcutaneous tissue in the upper arm (implantable). Glucose readings are transmitted wirelessly at 1–5-min intervals to a reader or a smartphone app. Some CGM devices transmit glucose data only when the user scans their sensor with a reader or smartphone app. This is referred to as intermittently scanned CGM (isCGM) or as FLASH glucose monitoring, referring in both cases to the FreeStyle Libre system.
The accuracy of CGM devices in comparison with reference plasma glucose measurements is well validated [3], such that several CGM devices are authorized by regulatory authorities to replace fingerstick SMBG testing for making diabetes treatment decisions, including insulin dosing (non-adjunctive use). In addition, a specific category of Class II device type, identified as an integrated CGM (iCGM) device [4, 5], has been created by the US Food and Drug Administration (FDA) for CGM devices that are suitable for use with certain digitally connected medical devices, including automated insulin delivery (AID) systems.
Currently available transcutaneous systems have sensors that have wear times from 6 to 14 days [6], after which a new sensor is applied. Implantable systems currently transmit glucose data for up to 180 days before replacement [7]. CGM sensors may be factory calibrated [6] or calibrated using a code provided with each sensor [8]. Alternatively, CGM sensors can require users to perform daily calibration using SMBG tests [9].
An important distinction is that CGM systems can be configured to operate as personal CGM devices, in which the user is able to see their glucose values on demand and to take action based on these values, or as professional CGM systems, which are blinded for the user and transmit glucose data that can be reviewed only by their diabetes HCP [10]. Here we review the evidence for wider application of CGM devices in the care of people with diabetes or glycemic dysregulation. In places the evidence is limited and a statement of unmet research needs is provided in Supplementary Table 1. All interpretations and recommendations meet ethics guidelines and conform with the 1964 Declaration of Helsinki. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Benefits of Using CGM in Daily Diabetes Care
A considerable body of evidence supports the use of CGM to transform health outcomes and quality of life for people with diabetes. In reality, this is paradigm shift in the treatment of people with diabetes, moving from reactive responses to the proactive prevention of high and low glucose levels.
When compared with SMBG, the use of CGM devices has been associated with lowered HbA1c in children and adults with type 1 diabetes mellitus (T1DM) [11,12,13,14,15,16,17,18], and in adults with type 2 diabetes mellitus (T2DM) treated either with insulin or a non-insulin therapy [19,20,21,22]. Both in T1DM and T2DM, use of CGM is associated with reduced risk of hypoglycemia [23,24,25]. Prospective trials have typically documented these glycemic benefits over periods up to 12 months; however, the COMISAIR prospective study demonstrated a persistent effect of the Dexcom G4 real-time CGM system over 3 years in adults with T1DM [14]. A second extended study of adults with T1DM started on CGM within 12 months of their diagnosis found that reductions in HbA1c were persistent with CGM use over a 7-year period [26]. A meta-analysis of 75 real-world studies involving the FreeStyle Libre system indicates that the glycemic benefits of using CGM persist for at least 2 years in adults with T2DM [27]. These evidence-based benefits of using CGM devices have confirmed their efficacy and place in the standard of care for people with T1DM or T2DM on insulin [28,29,30,31].
Importantly, CGM-derived metrics are now standardized for reporting [28,29,30]. Thus, using CGM compared to SMBG alone is associated with more time in range (TIR) with a glucose level of 70–180 mg/dL, less time above range (TAR) in hyperglycemia with a glucose level greater than 180 mg/dL, and less time below range (TBR) in hypoglycemia with a glucose level lower than 70 mg/dL, including nocturnal hypoglycemia, in people with T1DM or T2DM [23,24,25, 32]. CGM users also have less glucose variability, improved quality of life [33,34,35], and have fewer hospital admissions for acute diabetes events such as diabetic ketoacidosis (DKA) and severe hypoglycemia [36,37,38,39,40].
Along with the well-documented benefits for users and HCPs, there are also benefits for parents, carers, and partners of people with diabetes, the majority of whom feel positive about CGM [41]. Parents of children and adolescents with T1DM report improved quality of life and emotional well-being after their children started CGM [42, 43]. The reduced fear of hypoglycemia, especially at night, is a driver of improved well-being [44, 45]. Equally, using CGM is reported to reduce diabetes-specific conflict in families and facilitated parental involvement in diabetes management [43]. Carers for frail, older people with T1DM or T2DM also report greater ease of managing diabetes in this vulnerable group, with reduced concerns about hypoglycemia [46]. The ability to share glucose data is also seen as a positive aspect of using CGM by older adults and their caregivers [47]. All of these benefits are also reported by partners or spouses of people with diabetes [48].
Challenges for Using CGM in Diabetes Management
As with all medical technologies, using CGM systems is accompanied by challenges. Although current CGM devices are accurate across all ISF glucose ranges within 30–60 min after they are activated, they may be affected by a small number of substances, such as paracetamol, hydroxyurea, or high doses of vitamin C [49]. Because CGM sensors are on-body devices, they may be subject to physical stresses that can dislodge them or transiently affect their performance, such as artifacts from the user lying in a position that compresses the sensor and the tissue around it [50]. CGM sensors are also visible clues that the wearer has diabetes, which can become a focus for conscious or unconscious judgement and stigmatization, even from family and their HCPs [51]. Possibly the biggest challenge of using CGM is the huge amount of data that it provides, both during daily use and for review with HCPs. The user may overreact to changes in visible glucose levels or trends, with consequent micromanagement. The task of reviewing ambulatory glucose profile (AGP) reports can also be daunting, requiring education for users and HCPs (see below). However, research with CGM users and HCPs indicates that these challenges are not a major barrier to successful implementation, either for people with diabetes or their HCPs [42].
CGM in Guidelines for Diabetes Management
Use of CGM has rapidly become an accepted part of clinical guidelines for the treatment of diabetes. In the UK, the National Institute for Health and care Excellence (NICE) recommended access to isCGM for subgroups of people with T1DM on insulin therapy as early as 2017 [52] and current guidelines now recommend either isCGM or real-time CGM for all adults and children with T1DM and people with T2DM on intensive insulin therapy [53,54,55]. Diabetes technology was included in the American Diabetes Association (ADA) standards of medical care in diabetes for the first time only in 2019, at which point stand-alone CGM was suggested for “consideration” in children and adolescents with T1DM and indicated as a “useful tool” for adults with T1DM [56]. By 2022, the same guidelines simply state: “Use of CGM devices should be considered from the outset of the diagnosis of diabetes that requires insulin management” [57]. This has been echoed in the consensus guidelines for management of T1DM from the European Association for the Study of Diabetes (EASD) and the ADA [58], which indicate that CGM is the preferred option for glucose monitoring in newly diagnosed T1DM in adults, and should be considered for adults with established T1DM, even if they are meeting glycemic targets.
While recognizing and supporting the value of using CGM for children with T1DM [59, 60], the International Society for Pediatric and Adolescent Diabetes (ISPAD) does not make a specific recommendation to initiate CGM at the point of diagnosis for children with T1DM. We propose that this step should be taken, even in low-resource settings [61]. The long-term benefits of proactive glucose control with CGM for children with T1DM are compelling.
Similarly, CGM can be used as part of a targeted screening and monitoring strategy in individuals identified as at-risk for T1DM as a consequence of family history or detectable islet autoantibodies [62]. As they progress to stage 1, stage 2, and pre-symptomatic stage 3 in T1DM, use of CGM can detect abnormal glucose metabolism as individuals progress to stage 2. Proactive screening in this way can reduce the incidence of DKA and rates of hospitalization as symptoms become overt [62].
The 2022 ADA/EASD consensus guidelines for management of hyperglycemia in T2DM [63] also recommend the use of CGM for people with T2DM treated with insulin. An expert consensus group from the American Association of Clinical Endocrinology (AACE) published guidelines in 2021 that strongly recommended CGM for all persons with diabetes treated with intensive insulin therapy, defined as three or more injections of insulin per day or the use of an insulin pump [64]. In this context, real-time CGM was recommended over isCGM for people with diabetes and problematic hypoglycemia, because of the value of low-glucose alarms. However, second-generation isCGM systems already provided low- and high-glucose alarms at the point this recommendation was made [65], underlining the rapid pace of change in this aspect of diabetes care.
Most recently, Asia–Pacific (APAC) consensus recommendations [66] for the application of CGM in diabetes include initiating CGM as soon as possible after a diagnosis of T1DM and for people with T2DM on intensive insulin therapy and suboptimal glycemic control or high risk of problematic hypoglycemia. The APAC guidance also makes clear statements regarding daily or intermittent use of CGM for people with T2DM during Ramadan, for frail, older individuals with T2DM, pregnant women with pregestational T2DM, and women with gestational diabetes mellitus (GDM). Notably, the APAC guidelines include a process for interpreting AGP reports in APAC settings along with targets for core CGM metrics in adults with T1DM, T2DM, and in pregnancy for women with T1DM or T2DM [66].
CGM May be Used on a Day-to-Day or Intermittent Basis
Children and adult users of CGM in the management of T1DM are recommended to wear a glucose sensor on a daily basis, since clinical evidence in support of CGM has been collected in this way and reflects the need for people with T1DM on intensive insulin therapy to maintain control over fluctuating glucose levels and avoid potentially harmful episodes of hypoglycemia [11, 13, 24, 67]. CGM systems provide on-demand glucose readings alongside valuable information on the direction and rate of change (ROC) of glucose, indicated by trend arrows [68]. For people with T2DM on insulin therapy, the risks of hypoglycemia are also considerable [69] and daily use is supported by evidence [20, 25] and also recommended in guidelines (see above).
Daily use of isCGM or real-time CGM is now typically reimbursed by healthcare systems as part of care for people with T1DM and increasingly for people with T2DM on intensive insulin therapy. The benefits of using CGM in people with T2DM on basal-only regimens or non-insulin therapies are also demonstrated [21, 22, 40, 70, 71], but access to CGM is limited for these and other groups of people with diabetes because of the need to control costs, such that recommending daily use is impractical for most health services. Therefore, the intermittent use of CGM devices can be recommended as a practical alternative that is more realistic from a cost-impact perspective, in which the CGM device is used only for pre-specified periods during diabetes care.
Benefits of Intermittent Use of CGM
Intermittent use of CGM for people with T2DM has been proposed previously [72], to support management of poorly controlled patients on any therapeutic regimen, and is also recommended in ADA guidance of the use of technology in diabetes [57]. Use of intermittent or professional CGM should always be followed by a review with the person with diabetes, including any education required as part of changes to medication and/or lifestyle [57]. There is an unmet need for further supporting evidence on this aspect of CGM use.
A systematic review of 11 studies involving 5542 patients, 90% not on intensive insulin therapy, concluded that intermittent use of CGM was associated with reductions in HbA1c and body weight, as well as improved adherence to dietary plans and physical activity [73]. Periodic use of CGM in people with T2DM not on insulin therapy has shown reduced HbA1c and improved TIR [74, 75]. These changes are most significant for people with T2DM using CGM for 10 days at 3-month intervals, who also had a mean SMBG use at a frequency of 1.5 tests per day or more [75] over a 6-month prospective study period. In this study the intervention with CGM was non-blinded, allowing participants to see their glucose readings and trends.
The use of professional CGM, in which the CGM data is only available to the HCP, has also been tested in people with T2DM not on insulin therapy. A 2008 randomized controlled trial (RCT) demonstrated that a single 3-day application of blinded CGM, with follow-up review and education with a HCP, can lead to changes in behavior and reduced HbA1c in people with T2DM failing on non-insulin therapy [76]. Just as importantly, studies using blinded CGM even for 5 days have confirmed that approximately 50% of people with T2DM, including those on non-insulin therapy, experience frequent mild or clinically significant hypoglycemia [77] and this is asymptomatic in most cases. Such insights can allow for treatment adjustment focused on reducing the risk of hypoglycemia alongside overall glycemic control.
Intermittent application of CGM systems, either as part of unblinded or blinded use, is able to deliver glycemic information of value both to the person with T2DM and to their HCP, thereby facilitating improved glycemic control. Of clinical importance, this can be maintained through changes to lifestyle and periodic medication review and adjustment. No studies have examined the long-term application of intermittent CGM in T2DM.
Adapting Diabetes Care to the Cost of CGM Technology
Evidence on the cost-effectiveness of using CGM devices in diabetes care is limited. The per-unit costs of CGM sensors are higher than SMBG test strips and meters, which has created a perceived barrier to wider adoption. However, the long-term costs for daily CGM use, when factoring in savings against strip usage and costs for medical consultation and care of diabetes complications, are modelled as being cost-effective for people with T1DM [78,79,80,81]. In these analyses, much of the value assigned to CGM use was in the saved costs for reduced cumulative rates of diabetes complications and deaths in cardiovascular disease, ulcers and amputations, and renal disease. Although these complications are all part of long-term health outcomes in T2DM, a formal assessment for the cost impact of T2DM over the lifetime horizon is an unmet need. However, at least two studies have demonstrated that intermittent use of CGM in people with T2DM not on prandial insulin is a cost-effective intervention [82, 83], again based on lifetime reductions in treatment costs for diabetes complications. The periodic use of CGM in each of these studies was not similar, one at a high frequency [82], the other at a low frequency [83], suggesting that the optimal period between applications and glycemic assessment requires further study.
In order to minimize costs to healthcare providers we would argue that the most cost-effective use of intermittent CGM would favor the use of the FreeStyle Libre 2 or FreeStyle Libre 3 system. Glycemic outcomes data for the FreeStyle Libre system are comparable with those of real-time CGM systems, including reductions in HbA1c, improvements in TIR, and reductions in hypoglycemia [16, 25, 84]. The FreeStyle Libre sensors are factory calibrated and need no daily fingerstick calibration, which makes them suited to periodic application and adherence with use. They also may be more suited to intermittent use as they are easy to apply and do not require a transmitter, such that users have less to self-manage during occasional use. Critically, they have the lowest acquisition cost among currently available CGM devices and the longest on-body wear time, with each sensor lasting 14 days. The FreeStyle Libre 2 and FreeStyle Libre 3 systems are also enabled with optional alarms that may reinforce the educational value for the user during 14-day intermittent use. Freestyle Libre sensors can also be used as part of professional CGM, although different healthcare territories have selective availability of this option.
Optimizing the Value of CGM in Modern Diabetes Care
Diabetes care using CGM has typically focused on addressing the unmet needs for day-to-day glycemic control. This has meant focusing on reducing HbA1c for people with poorly controlled diabetes, as well as reducing the risks of symptomatic and severe hypoglycemia for people on insulin or insulinotropic therapies. There is sufficient data to support the use of CGM in a much wider group of people with diabetes such that it meets a range of needs at different times throughout their lives with diabetes. These are all separate from the management of T1DM or T2DM on insulin therapy, in which daily use of CGM is indicated and supported by guidelines.
At the Point of Diagnosis of T2DM
There are many pathophysiological changes to glucose homeostasis that result in persistent hyperglycemia and a diagnosis of T2DM. Ultimately, this also means that T2DM is a very heterogeneous disease [85]. Over the last few years, analysis of glucose profiles for people with T2DM has suggested that some of this heterogeneity can be mapped to glucotypes, based on patient characteristics and CGM-defined glycemic metrics [85, 86]. It has been proposed that some of these glucotypes may be predictive of future complications [85]. At the time of diagnosis, it therefore makes sense to establish as much as possible about the baseline glycemic profile for any person with T2DM, such that a diabetes management plan can be put in place as early as possible that meets their individual treatment needs. Intermittent use of CGM should be used as soon possible after diagnosis to establish these baseline glucose parameters for each person with T2DM, against which subsequent treatment decisions may be compared and disease progression monitored.
Monitoring Proactive Treatment Intensification
Clinical inertia is a term that defines the reticence of HCPs to initiate or intensify therapy for people with T2DM in a timely manner, as recommended by evidence-based clinical guidelines [87,88,89]. Although a change of therapy in T2DM is indicated if HbA1c goals are not achieved after 3 months on the current regimen [90], reported times to treatment intensification are considerably higher, with the median time to treatment intensification after an above-target HbA1c test reading being measured in years rather than months [91], and this inertia may be considerably prolonged as the number of antidiabetic drugs in the treatment plan rises [91]. Such clinical inertia is linked to the increased incidence of microvascular and macrovascular disease in T2DM [92, 93], as well as significantly increased costs related to diabetes complications [94, 95].
It does not make sense to ignore the value of CGM as part of treatment intensification for people with T2D, particularly as fear of hypoglycemia is known to be a significant factor in clinical inertia for patients and clinicians alike [96]. Use of CGM in people with T2DM on basal-only insulin is associated with improvements in HbA1c while reducing hypoglycemia [19].
A retrospective analysis of large healthcare claims datasets indicate that use of a CGM device by people with T2DM is associated with more-timely treatment intensification compared to those using fingerprick SMBG testing alone [97]. All treatment choices were facilitated in this analysis, including starting and intensifying insulin therapy. Although this analysis applied to people with T2DM on daily CGM use, it can be argued that intermittent use at the point of treatment intensification can support the same outcomes. A prospective study using professional CGM in 68 people with T2D on any treatment regimen [98] showed that 14-days use of professional CGM in a primary care setting, followed by a review with the HCP and any treatment recommendations, is effective at lowering HbA1c, increasing TIR, and facilitating treatment intensification. The improvement in glycemic management was accomplished by a combination of lifestyle counseling and medication intensification, rather than an increase in the number of medications. Use of a second application of professional CGM after 3–6 months in a subset of participants was associated with more intensive treatment modification [99]. Together with the data on cost-efficacy for intermittent CGM in T2DM [82, 83], these data clearly indicate that intermittent CGM can be used to support treatment intensification in T2DM and this can be managed in a primary care or clinical pharmacy setting.
CGM Coaching and Motivational Support for Behavioral Change in People with Diabetes
The glycemic benefits of intermittent CGM are connected to its impact on the overall behavior of people with T1DM or T2DM. Once they can see how the daily activities associated with diet, physical activity, and adherence to treatment affect glucose levels, it makes sense to adapt these activities even after the CGM has been withdrawn. Demonstrating this holistic effect in a study setting is not easy and there are only a small number that address this. Using isCGM has been linked to improved self-awareness of food consumption for people with T1DM, including increased confidence in food choices in relation to their expectations for diabetes management [100].
Lifestyle counseling following application of professional CGM was credited with the glycemic changes in people with T2DM, as much as medication intensification [98], and intermittent use of CGM has been associated with improved adherence to dietary plans, improved healthy eating, and physical activity [73, 101]. When used as an adjunct to diabetes and lifestyle education for people with T2DM, intermittent CGM has resulted in greater improvements than education alone [102], and the use of motivational text messaging has been successful in promoting lifestyle changes and reduction of glycemia in people recently diagnosed with T2DM who used personal CGM for a limited time [103]. We suggest therefore that such “CGM coaching” is a valid tool as part of the diabetes self-management education and support (DSMES) intervention that is identified as being as equally critical as pharmacological treatment for people with T2DM [63]. Further objective research in this context is warranted.
CGM is Effective in Daily Management of T2DM on Insulin Regimens
It is accepted that people with T2DM on intensive insulin therapy benefit from using CGM devices in the same way that has been shown for people with T1DM. This includes reduced HbA1c [20, 84], reduced hypoglycemia [25, 84], and fewer acute diabetes events leading to hospital admission [38, 104]. Consequently, the benefits of using CGM in T2DM on intensive insulin therapy are increasingly reflected in guidelines for management of T2DM [54, 63, 105] as part of a holistic care plan. Use of premixed insulins may still be recommended to improve adherence with intensive treatment in T2DM. Although no prospective studies have examined the use of CGM in people using premixed insulins, the overall benefits and risks of therapy with premixed insulins are comparable with adding a bolus insulin to basal therapy [106].
Less-intensive basal-only insulin therapy is recommended for people with T2DM who are failing on oral therapies [63]. Initiation of basal insulin is associated with episodes of problematic hypoglycemia and is the second most common reason given by people with T2DM for interruption or discontinuation of basal insulin therapy [107, 108]. For people with T2DM on basal insulin therapy, the MOBILE RCT has shown that using CGM can lower HbA1c and reduce both time in hyperglycemia with glucose level greater than 250 mg/dL (13.9 mmol/L) and hypoglycemia event rates over an 8-month period, compared to a control group using SMBG testing alone [19]. Notably, discontinuing CGM use in this group resulted in the loss of half of the gains in TIR within 6 months of ceasing use [109]. These data confirm the results of retrospective studies showing a significant reduction in HbA1c for people with T2DM on basal insulin therapy [70, 110]. Together, these outcomes support the proposition that people with T2DM on basal insulin regimens should be provided with access to CGM devices for daily use as is accepted for those on intensive insulin regimens.
Using CGM in Cystic Fibrosis-Related Diabetes
Cystic fibrosis-related diabetes (CFRD) is one of the most common extrapulmonary comorbidities among people with CF, occurring in up to 50% of adults [111]. CFRD is a form of diabetes caused by relative insulin insufficiency secondary to destruction of pancreatic islets [112, 113]. CFRD is associated with worse clinical outcomes, decreased pulmonary function, and higher mortality rates [114]. The reference standard screening for CFRD is annual oral glucose tolerance testing (OGTT), but when CGM has been applied contemporaneously with OGTT testing, CGM has detected CFRD at a higher frequency [115] and identified glucose abnormalities [116]. Notably, CGM metrics of hyperglycemia and glycemic variability are superior to HbA1c in differentiating between people with CF who have CFRD and those without [117]. Furthermore, when compared with SMBG, use of CGM in people with CFRD is associated with improved glycemic control and significantly reduced HbA1c [118].
Given that insulin therapy is an accepted intervention in CFRD, the use of CGM to confirm diagnosis and establish the need for insulin treatment has been proposed [119]. Since up to 96% of school-age children and 85% of adults with CFRD are treated with insulin [120], the application of daily CGM for people with CFRD should be considered in this group. No longitudinal studies have been undertaken to establish whether using CGM in CFRD is associated with improved pulmonary or non-pulmonary outcomes in CF. The role of CGM for women with CFRD during pregnancy is discussed later in this article.
Use of CGM in People with T2DM Not on Insulin Therapy
Evidence shows that people with T2DM not on insulin therapy can significantly reduce their HbA1c using CGM devices [21, 22, 121]. This reduction is greater for those with a higher HbA1c level at the point of initiation [21]. Glycemic variability is also reduced in people with T2DM on non-insulin therapy using CGM compared to SMBG [22]. Similarly, use of CGM amongst people can be associated with reduced acute diabetes events requiring hospital attendance or admission [71].
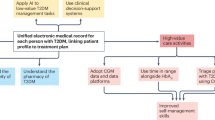
In real-world healthcare economies, people with T2DM not on insulin comprise the majority of the diabetes treatment population, and recommending daily access to CGM systems for this group is impractical because of the cost impact. Therefore, the intermittent use of CGM devices can be recommended at regular intervals or during treatment change, as discussed previously. Therefore, for people with stable T2DM on non-insulin therapies unblinded CGM sensors should be used every 3 months as standard of care. In this way it will be possible to (1) evaluate treatment responses and achievement of goals; (2) adjust therapy as necessary; (3) evaluate risks for microvascular and cardiometabolic complications; and (4) reinforce patient education and diabetes self-management skills (CGM coaching). This is summarized in Fig. 1.
Importance of CGM in T2DM in the Elderly and Vulnerable
The incidence of T2DM amongst the elderly population is significant, particularly in high- and middle-income countries [122]. Despite this, there are relatively few studies on the use of CGM in elderly populations and this is an important gap. The REPLACE RCT in people with T2DM on intensive insulin therapy found that study participants aged 65 years or older had a 56% reduction in TBR 70 mg/dL, which was comparable to subjects younger than 65 years old [25]. A subgroup analysis of the DIAMOND study showed that, in adults aged 60 years or older with T2DM on intensive insulin therapy [20], improvements in HbA1c and reduced glycemic variability obtained using daily CGM were not different from those in younger adults. Bao and colleagues reported that people with T2DM aged 65 years or older on basal insulin therapy were able to improve TIR and reduce hypoglycemia after starting traditional CGM, at least as significantly as younger adults [123]. The outcomes of the RELIEF study on use of isCGM in adults with T2DM aged 65 years or older on intensive insulin therapy have shown that hospital admissions for acute diabetes events were reduced by 34% and 40% in the 12 and 24 months following initiation of isCGM, respectively [124].
Use of blinded CGM [125] and retrospective insurance claims analysis [126] have confirmed that hypoglycemia is frequent amongst older populations with T2DM, including those on non-insulin therapy, and those with elevated HbA1c. It is also evident that the risk of severe or fatal hypoglycemia increases considerably in elderly individuals with diabetes who are treated with insulinotropic medications [127,128,129], and the risk of falls and fractures amongst elderly people with T2DM treated with insulin is increased compared to those only on oral medication [130, 131]. Hypoglycemia in elderly people with T2DM is also associated with increased incidence of cardiovascular events, dementia, and death [131]. The avoidance of hypoglycemia has therefore been suggested as a greater priority than minimizing HbA1c amongst elderly people with T2DM [132].
The Imperative for De-intensifying Treatment in Elderly People with Diabetes
The goal of symptom control and maintaining quality of life as people reach advanced age creates a need for treatment de-intensification in a safe manner [133]. Deprescribing sulfonylurea drugs is a clear target [133, 134], particularly in elderly people with T2DM with more complications and comorbidities. Clinical management of diabetes in elderly and frail people with diabetes is hampered by the paucity of studies that include this population. It is clear that de-prescribing of medication in elderly people with diabetes required judicious clinical judgement, which can be facilitated with the use of CGM to maintain awareness of glycemic changes. Since the feasibility and acceptability of CGM in very elderly adults up to 91 years old has also been demonstrated [135], the case for wider access and application of CGM in older and elderly people with T2DM is clear (Fig. 1).
Cognitive and Mental Health Concerns in Elderly People with Diabetes
The phenomenon of cognitive decline is associated with aging in the general population [136], and risk of developing dementia is increased in T2DM [137]. The features of cognitive decline can reduce the ability and engagement of elderly people with T2DM to manage their basic self-care tasks [138], with the burden of care then transferring to carers and HCPs. Cognitive deficits also contribute to an increased frequency of depression-related symptoms, poorer adherence to treatment, increased risk of hypoglycemia, and ultimately to poorer prognosis [139]. In this context, the needs of older adults with T2DM and their caregivers can benefit from the use of CGM in this population. Daily use of CGM can help elderly people with T2DM and their carers to manage medication and glycemic risks, with as little extra burden as possible. In this way, application of CGM can maintain quality of life for this patient group and limit the risks of functional dependency [140].
Controlling Hyperglycemia in Acute Care Settings
The benefits of using CGM in acute and critical care settings has not been well explored to date, although the impact of glycemic dysregulation for people without diabetes in hospital is becoming better documented. Hyperglycemia in this context is stress induced as a consequence of an inflammatory and adrenergic response, when counterregulatory hormones are released that lead to insulin resistance and reduced insulin secretion. Glucagon release and glycogenolysis can also occur [141, 142].
Stress-induced hyperglycemia is associated with risk of death or poor outcomes after acute myocardial infarction (AMI) [143] and intensive control of hyperglycemia in people with diabetes is associated with reduced mortality in the 12 months following an AMI [144]. This effect is most pronounced in people with T2DM naïve to insulin prior to admission to hospital [144], and can persist up to 8 years following intervention [145]. However, the risks of hypoglycemia following intensive control of hyperglycemia following AMI have also been proposed to confound these beneficial effects of reducing hyperglycemia for people with T2DM following AMI [146, 147].
The association between hyperglycemia and risk of stroke is well established in people with T2DM [148]. Poorer outcomes for people suffering acute stroke are even more linked to hyperglycemia [149], such that “stress hyperglycemia” in patients with stroke and without a diagnosis of diabetes is predictive of a worse prognosis [150]. The link between hyperglycemia and stroke is proposed to involve the pro-inflammatory consequences of persistent high glucose [151] and American Stroke Association guidelines recommend establishing blood glucose levels in the range of 140–180 mg/dL (7.8–10.0 mmol/L) as soon as possible following a stroke and to avoid hypoglycemia [152].
Hyperglycemia is a common and significant adverse consequence of several established cancer therapeutic classes, including immune checkpoint inhibitors (ICIs), phosphatidylinositol 3-kinase (PI3K) inhibitors, mammalian target of rapamycin (mTOR) inhibitors, 5-fluorouracil (5-FU) analogues, and glucocorticoids [153]. The mechanism can be related to drug-mediated insulin resistance but not always [153]. This hyperglycemia has been associated with reduced efficacy of cancer therapy [154]. Use of CGM data has also shown that hyperglycemia associated with in-hospital treatment is also associated with longer hospital stays and worsened prognosis [155, 156]. From a clinical perspective, hyperglycemia is established as an independent risk factor for earlier cancer recurrences and higher mortality rates [157].
As well as the specific conditions discussed above, management of a much wider group of acute surgical patients with comorbid T2DM is also subject to guidance on minimizing hyperglycemia in the hospital setting because of the increased risks for surgical-site infections [158, 159] and increased costs [160].
Given the proven role CGM in reducing hyperglycemia with reduced risk of severe hypoglycemia for people with T2DM on intensive therapies [25, 161], the use of CGM as part of acute management for cardiometabolic, neurological, and oncological conditions must be strongly considered.
Post-Transplant Hyperglycemia and Diabetes
Recipients of organ transplants can develop post-transplantation diabetes (PTDM) in the first year after transplantation as a consequence of immunosuppressive therapy and β-cell dysfunction. PTDM is common, affecting up to 30% of kidney [162], heart [163], liver [164], and lung transplant [164] patients who were non-diabetic pre-transplant. Although PTDM is not thought to be associated with microvascular and macrovascular complications, it is associated with increased risk of cardiovascular morbidity and mortality and graft loss, and lower rates of survival in patients with PTDM [165].
The adverse consequences of PTDM mean that intensive glucose monitoring and management is necessary in the immediate period after transplantation surgery. This can be achieved using CGM, which is straightforward for postsurgical teams to apply and for graft recipients to self-manage during the weeks and months as outpatients.
Use of CGM in Chronic Kidney Disease (CKD)
There is only limited evidence that progression of diabetes-related kidney disease may be slowed using CGM to optimize TIR [166, 167]. However, several studies using CGM have shown that patients with T2DM and stage 3–5 CKD have lower average glucose levels during and immediately after hemodialysis as compared to days not on dialysis [168, 169]. Consequently, it is important to match their basal insulin doses to their glycemic needs during this period, to avoid an increased frequency of hypoglycemia. In this context, CGM devices can reveal the important daily glycemic patterns for people with T2DM and CKD on hemodialysis and help them manage daily diet and physical activity. Another aspect of CKD and other metabolic diseases is that HbA1c can be compromised as a marker of overall glycemia, because it is sensitive to non-glycemic changes in red blood cell volume and turnover, or protein glycation rates. Therefore, it has been proposed that the glucose management indicator (GMI) [170], a CGM-derived marker, can be used to evaluate overall glucose exposure for people in whom HbA1c may be an unreliable [171, 172].
The Important Role of CGM in Pregnancy
Despite advances in antenatal diabetes care, 60% of babies born to women with T1DM are large for gestational age (LGA), which is associated with increased rates of obstetric and neonatal complications [173, 174]. Pregnant women with T2DM have higher than expected rates of perinatal death and increased rates of LGA deliveries [173]. For women without diabetes prior to conceiving, GDM [175] is the dominant condition among pregnancies complicated by diabetes, affecting 3–10% of all pregnancies and is associated with risk of fetal macrosomia, shoulder dystocia, birth trauma, and caesarean section [175]. It is important to note that GDM is a metabolic disorder distinct from pregestational diabetes. It is less severe and typically resolves after giving birth. The benefits of using CGM in GDM need to be better researched. However, data obtained using CGM in women with GDM show that significant daytime glucose variability and elevated mean glucose levels overnight are associated with increased risk of fetal complications, including LGA, in GDM [176,177,178]. The high rate of fetal growth after the 30th week of gestation forces the rapid implementation of effective therapy for GDM, which is usually diagnosed around the 26th to 28th week of gestation.
Meta-analyses of studies investigating the impact of CGM on GDM pregnancies have concluded that women with GDM better implemented dietary recommendations and achieved better glycemic control when using CGM, compared to women using SMBG alone [179, 180]. The incidence of LGA births was also reduced for women with GDM who used CGM, compared to those using SMBG [179, 180].
Amongst all pregnancies affected by diabetes, LGA infants are predisposed to developing obesity, T2DM, and cardiovascular disease, persisting into adulthood [181, 182]. As therapies for CF are offering greater health and longevity, women with CF are increasingly pursuing pregnancy [183]. The prevalence of GDM or CFRD in this group is high, with up to 66% of pregnancies affected [184] and with associated risks for LGA and perinatal complications [183].
Application of CGM during pregnancy has shown that during the critical stages of early pregnancy women with pregestational T1DM or T2DM spend only 50% of each day (12 h) with glucose levels in the target glucose range 70–140 mg/dL (3.9–7.8 mmol/L) [185]. The CONCEPTT trial [186] in 215 pregnant women with T1DM, using a target glucose range of 63–140 mg/dL (3.5–7.8 mmol/L), showed that TIR increased by approximately 10% (2 h 24 min/day) from the first to the third trimester. The CONCEPTT trial also showed that use of CGM helps women with T1DM improve their %TIR during pregnancy compared to controls using SMBG (68% vs 61%; 16 h 19 min vs 14 h 38 min/day) [186]. This improvement in glycemia was achieved without increased maternal hypoglycemia.
A retrospective analysis [187] of CGM data from both the CONCEPTT trial [186] and a Swedish retrospective study [188] on a total of 386 pregnancies found that women with T1DM who went on to have LGA deliveries had significantly lower time in the pregnancy target glucose range from around 6 to 8 weeks of gestation, compared to women with T1DM who delivered normal sized infants. Ultimately, normal birth weight for women with T1DM is associated with achieving significantly lower mean daily glucose concentration and higher time in target glucose range from before the end of the first trimester [187]. The relevance of CGM in this context is further underscored by data indicating increased glucose variability is an independent risk factor for delivering LGA infants for women with pregestational diabetes [189, 190], and that use of isCGM is associated with a lower rate of spontaneous abortion in pregnant women with T1DM, when compared to SMBG [191].
The outcome of these studies in T1DM has led to international consensus guidelines which propose that pregnant women with pregestational diabetes or gestational diabetes spend greater than 70% of time with glucose between 63 and 140 mg/dL (3.5–7.8 mmol/L) [30].
Specific studies on using CGM or isCGM in pregnancy in T2DM and in GDM are planned to complete this picture [192]. Overall, the need to meet CGM-derived glucose targets for maternal glycemia from early pregnancy [187] emphasizes the need to use CGM devices in women either with pregestational diabetes or GDM, including in women with CFRD, from the earliest possible moment in pregnancy.
Modern Management of Diabetes with CGM: A Challenge for Healthcare Professionals and Healthcare Services
Expanding the application of CGM systems for management of people with T2DM and beyond will require a considerable adjustment in diabetes service delivery. Key among these is education for HCPs, particularly in understanding the heterogeneity of T2DM and the diverse patient profiles that must be managed [85, 86]. Similarly, the high value of CGM in a much larger population of users is due to its utility for remote monitoring, reducing the need for in-clinic consultations and for streamlining workflows [193]. A concern in this context is a 2018 survey of primary care physicians across the EU, which found that 89% did not engage with telemedicine solutions with their patients, that 81% did not use it with other HCPs, and that 51% were either unaware that telemedicine education was available or did not access it [194]. This landscape is likely to have changed as a consequence of the COVID-19 pandemic, during which telemedicine became the predominant mode of diabetes consultations [195]. Although limited, the available evidence suggests that telemedicine is not inferior to face-to-face visits in diabetes care [195, 196], and that primary care teams and patients have embraced it [197, 198].
The Expanding Frontier of Modern Diabetes Care: A Map of Unexplored Opportunity
We have reviewed the evidence base for use of CGM in people with diabetes and also non-diabetic individuals who are challenged by hyperglycemia in acute circumstances. This has allowed us to propose the map of applications outlined in Fig. 2. The number of potential applications for using CGM for managing glucose dysregulation is far greater than those agreed in consensus guidelines and for which reimbursement is typically provided by national health services. Seizing this opportunity will require the concerted efforts of HCPs and professional societies, to create and agree the necessary guidelines for the indications and frequency of use of CGM in the diverse conditions described. Equally, there is a significant unmet need for evidence that the proposed use of CGM can be associated with improved outcomes in each case. This evidence will be the foundation on which budget stakeholders will assess the cost–benefit for expanding use of CGM and recommend access.
Conclusions
The evidence on which to recommend inclusion of CGM technology as the standard of care for all people with T1DM and T2DM is available, including for people with T2DM on non-insulin therapies. Many other clinical scenarios are also deserving of the application of CGM in order to manage acute episodes of glycemic dysregulation in people without established diabetes. The value of CGM as an intermittent intervention at predefined points in the patient journey is key to its widespread and cost-effective use. Extracting the untapped value of CGM technologies in diabetes care is a significant challenge for healthcare teams and healthcare economies, and will require a paradigm shift in attitudes, education, and service design.
References
Freckmann G, Pleus S, Grady M, Setford S, Levy B. Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J Diabetes Sci Technol. 2019;13:575–83. https://doi.org/10.1177/1932296818812062.
Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787–94. https://doi.org/10.1089/dia.2014.0378.
Ajjan RA, Cummings MH, Jennings P, Leelarathna L, Rayman G, Wilmot EG. Accuracy of flash glucose monitoring and continuous glucose monitoring technologies: implications for clinical practice. Diabetes Vasc Dis Re. 2018;15:175–84. https://doi.org/10.1177/1479164118756240.
Garg SK, Akturk HK. A new era in continuous glucose monitoring: food and drug administration creates a new category of factory-calibrated nonadjunctive, interoperable class II medical devices. Diabetes Technol Ther. 2018;20:391–4. https://doi.org/10.1089/dia.2018.0142.
Bailey TS, Alva S. Landscape of continuous glucose monitoring (CGM) and integrated CGM: accuracy considerations. Diabetes Technol Ther. 2021;23:5–11. https://doi.org/10.1089/dia.2021.0236.
Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2020. https://doi.org/10.1177/1932296820958754.
Joseph JI. Review of the long-term implantable senseonics continuous glucose monitoring system and other continuous glucose monitoring systems. J Diabetes Sci Technol. 2021;15:167–73. https://doi.org/10.1177/1932296820911919.
Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20:428–33. https://doi.org/10.1089/dia.2018.0143.
van der Linden J, Welsh JB, Walker TC. Sustainable use of a real-time continuous glucose monitoring system from 2018 to 2020. Diabetes Technol Ther. 2021;23:508–11. https://doi.org/10.1089/dia.2021.0014.
Raviteja KV, Kumar R, Dayal D, Sachdeva N. Clinical efficacy of professional continuous glucose monitoring in improving glycemic control among children with type 1 diabetes mellitus: an open-label randomized control trial. Sci Rep. 2019;9:6120. https://doi.org/10.1038/s41598-019-42555-6.
Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317:379–87. https://doi.org/10.1001/jama.2016.19976.
Aleppo G, Ruedy KJ, Riddlesworth TD, et al. REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care. 2017;40:538–45. https://doi.org/10.2337/dc16-2482.
Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371–8. https://doi.org/10.1001/jama.2016.19975.
Šoupal J, Petruželková L, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care. 2019;43:37–43. https://doi.org/10.2337/dc19-0888.
Campbell FM, Murphy NP, Stewart C, Biester T, Kordonouri O. Outcomes of using flash glucose monitoring technology by children and young people with type 1 diabetes in a single arm study. Pediatr Diabetes. 2018;19:1294–301. https://doi.org/10.1111/pedi.12735.
Leelarathna L, Evans ML, Neupane S, et al. Intermittently scanned continuous glucose monitoring for type 1 diabetes. New Engl J Med. 2022. https://doi.org/10.1056/nejmoa2205650.
Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155–62. https://doi.org/10.1007/s00125-012-2708-9.
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17–22. https://doi.org/10.2337/dc09-1502.
Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin. JAMA. 2021;325:2262–72. https://doi.org/10.1001/jama.2021.7444.
Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Ann Intern Med. 2017;167:365. https://doi.org/10.7326/m16-2855.
Wright EE, Kerr MSD, Reyes IJ, Nabutovsky Y, Miller E. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184–9. https://doi.org/10.2337/ds20-0069.
Wada E, Onoue T, Kobayashi T, et al. Flash glucose monitoring helps achieve better glycemic control than conventional self-monitoring of blood glucose in non-insulin-treated type 2 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2020;8:e001115. https://doi.org/10.1136/bmjdrc-2019-001115.
Riddlesworth T, Price D, Cohen N, Beck RW. Hypoglycemic event frequency and the effect of continuous glucose monitoring in adults with type 1 diabetes using multiple daily insulin injections. Diabetes Ther. 2017;8:947–51. https://doi.org/10.1007/s13300-017-0281-4.
Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254–63. https://doi.org/10.1016/s0140-6736(16)31535-5.
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J-P, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter open-label randomized controlled trial. Diabetes Ther. 2017;8:55–73. https://doi.org/10.1007/s13300-016-0223-6.
Champakanath A, Akturk HK, Alonso GT, Snell-Bergeon JK, Shah VN. Continuous glucose monitoring initiation within first year of type 1 diabetes diagnosis is associated with improved glycemic outcomes: 7-year follow-up study. Diabetes Care. 2022;45:750–3. https://doi.org/10.2337/dc21-2004.
Evans M, Welsh Z, Seibold A. Reductions in HbA1c with flash glucose monitoring are sustained for up to 24 months: a meta-analysis of 75 real-world observational studies. Diabetes Ther. 2022. https://doi.org/10.1007/s13300-022-01253-9.
Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40:1622–30. https://doi.org/10.2337/dc17-1624.
Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–40. https://doi.org/10.2337/dc17-1600.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019. https://doi.org/10.2337/dci19-0028.
Beck RW, Bergenstal RM. Continuous glucose monitoring for type 2 diabetes: how does it compare with type 1 diabetes? Diabetes Technol Ther. 2022;24:153–6. https://doi.org/10.1089/dia.2021.0374.
Bergenstal RM, Mullen DM, Strock E, Johnson ML, Xi MX. Randomized comparison of self-monitored blood glucose (BGM) versus continuous glucose monitoring (CGM) data to optimize glucose control in type 2 diabetes. J Diabetes Complicat. 2022;36: 108106. https://doi.org/10.1016/j.jdiacomp.2021.108106.
Díez-Fernández A, Rodríguez-Huerta MD, Mirón-González R, Laredo-Aguilera JA, Martín-Espinosa NM. Flash glucose monitoring and patient satisfaction: a meta-review of systematic reviews. Int J Environ Res Pu. 2021;18:3123. https://doi.org/10.3390/ijerph18063123.
Gilbert TR, Noar A, Blalock O, Polonsky WH. Change in hemoglobin A1c and quality of life with real-time continuous glucose monitoring use by people with insulin-treated diabetes in the landmark study. Diabetes Technol Ther. 2021;23:35–9. https://doi.org/10.1089/dia.2020.0666.
Polonsky WH, Hessler D, Ruedy KJ, Beck RW. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the diamond randomized clinical trial. Diabetes Care. 2017;40:170. https://doi.org/10.2337/dc17-0133.
Charleer S, Mathieu C, Nobels F, et al. Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: a real-world study. J Clin Endocrinol Metab. 2018;103:1224–32. https://doi.org/10.1210/jc.2017-02498.
Charleer S, Block CD, Huffel LV, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43:389–97. https://doi.org/10.2337/dc19-1610.
Roussel R, Riveline J-P, Vicaut E, et al. Important drop rate of acute diabetes complications in people with type 1 or type 2 diabetes after initiation of flash glucose monitoring in France: the RELIEF study. Diabetes Care. 2021. https://doi.org/10.2337/dc20-1690.
Riveline J-P, Roussel R, Vicaut E, et al. Reduced rate of acute diabetes events with flash glucose monitoring is sustained for two-years after initiation: extended outcomes from the RELIEF study. Diabetes Technol Ther. 2022. https://doi.org/10.1089/dia.2022.0085.
Guerci B, Roussel R, Levrat-Guillen F, et al. Important decrease in hospitalizations for acute diabetes events following FreeStyle Libre® system initiation in people with type 2 diabetes on basal insulin therapy in France. Diabetes Technol Ther. 2022. https://doi.org/10.1089/dia.2022.0271.
Barnard K, Crabtree V, Adolfsson P, et al. Impact of type 1 diabetes technology on family members/significant others of people with diabetes. J Diabetes Sci Technol. 2016;10:824–30. https://doi.org/10.1177/1932296816645365.
Beasant L, Cullen F, Thomas E, et al. Flash glucose monitoring in young people with type 1 diabetes—a qualitative study of young people, parents and health professionals: ‘it makes life much easier.’ BMJ Open. 2023;13:e070477. https://doi.org/10.1136/bmjopen-2022-070477.
Boucher SE, Aum SH, Crocket HR, et al. Exploring parental perspectives after commencement of flash glucose monitoring for type 1 diabetes in adolescents and young adults not meeting glycaemic targets: a qualitative study. Diabet Med. 2020;37:657–64. https://doi.org/10.1111/dme.14188.
Ng SM, Moore HS, Clemente MF, Pintus D, Soni A. Continuous glucose monitoring in children with type 1 diabetes improves well-being, alleviates worry and fear of hypoglycemia. Diabetes Technol Ther. 2019;21:133–7. https://doi.org/10.1089/dia.2018.0347.
Ng SM, Dearman S, Fisher M, Mushtaq T, Randell T. Paediatric society and hyperinsulinism charity national surveys on CGM access for patients with recurrent hypoglycaemia. J Endocr Soc. 2023;7:21. https://doi.org/10.1210/jendso/bvad021.
Mattishent K, Lane K, Salter C, et al. Continuous glucose monitoring in older people with diabetes and memory problems: a mixed-methods feasibility study in the UK. BMJ Open. 2019;9:e032037. https://doi.org/10.1136/bmjopen-2019-032037.
Allen NA, Litchman ML, Chamberlain J, Grigorian EG, Iacob E, Berg CA. Continuous glucose monitoring data sharing in older adults with type 1 diabetes: pilot intervention study. JMIR Diabetes. 2022;7:e35687. https://doi.org/10.2196/35687.
Polonsky WH, Fortmann AL. Impact of real-time CGM data sharing on quality of life in the caregivers of adults and children with type 1 diabetes. J Diabetes Sci Technol. 2020. https://doi.org/10.1177/1932296820978423.
Battelino T, Alexander CM, Amiel SA, et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 2022. https://doi.org/10.1016/s2213-8587(22)00319-9.
Mensh BD, Wisniewski NA, Neil BM, Burnett DR. Susceptibility of interstitial continuous glucose monitor performance to sleeping position. J Diabetes Sci Technol. 2013;7:863–70. https://doi.org/10.1177/193229681300700408.
Schabert J, Browne JL, Mosely K, Speight J. Social stigma in diabetes: a framework to understand a growing problem for an increasing epidemic. Patient. 2013;6:1–10. https://doi.org/10.1007/s40271-012-0001-0.
FreeStyle Libre for glucose monitoring. Medtech innovation briefing [MIB110] 2017.
Type 1 diabetes in adults: diagnosis and management NICE guideline Published: 26 August 2015. Last updated: 17 August 2022. https://www.nice.org.uk/guidance/ng17.
Type 2 diabetes in adults: management NICE guideline. Published: 2 December 2015. Last updated: 29 June 2022. https://www.nice.org.uk/guidance/ng28.
Diabetes (type 1 and type 2) in children and young people: diagnosis and management NICE guideline. Published: 1 August 2015. Last updated: 11 May 2023. https://www.nice.org.uk/guidance/ng18.
American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes—2019. Diabetes Care 2018;42:71–80. https://doi.org/10.2337/dc19-s007.
American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 7. Diabetes Technology: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021;45:S97–112. https://doi.org/10.2337/dc22-s007.
Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2021;44:2589–625. https://doi.org/10.2337/dci21-0043.
Tauschmann M, Forlenza G, Hood K, et al. ISPAD clinical practice consensus guidelines 2022: diabetes technologies: glucose monitoring. Pediatr Diabetes. 2022;23:1390–405. https://doi.org/10.1111/pedi.13451.
Bock M, Codner E, Craig ME, et al. ISPAD Clinical Practice Consensus Guidelines 2022: glycemic targets and glucose monitoring for children, adolescents, and young people with diabetes. Pediatr Diabetes. 2022;23:1270–6. https://doi.org/10.1111/pedi.13455.
Virmani A, Brink SJ, Middlehurst A, et al. ISPAD Clinical Practice Consensus Guidelines 2022: management of the child, adolescent, and young adult with diabetes in limited resource settings. Pediatr Diabetes. 2022;23:1529–51. https://doi.org/10.1111/pedi.13456.
Besser REJ, Bell KJ, Couper JJ, et al. Stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2022. https://doi.org/10.1111/pedi.13410.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022. https://doi.org/10.1007/s00125-022-05787-2.
Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology clinical practice guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract. 2021;27:505–37. https://doi.org/10.1016/j.eprac.2021.04.008.
Virdi N. Response to American Association of clinical endocrinology clinical practice guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract. 2021;27:1062. https://doi.org/10.1016/j.eprac.2021.07.006.
Kong APK, Lim S, Yoo SH, et al. Asia-Pacific consensus recommendations for application of continuous glucose monitoring in diabetes management. Diabetes Res Clin Pract. 2023. https://doi.org/10.1016/j.diabres.2023.110718.
JDRF CGM Study Group. JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther. 2008;10:310–21. https://doi.org/10.1089/dia.2007.0302.
Ziegler R, von Sengbusch S, Kröger J, et al. Therapy adjustments based on trend arrows using continuous glucose monitoring systems. J Diabetes Sci Technol. 2019. https://doi.org/10.1177/1932296818822539.
Akram K, Pedersen-Bjergaard U, Borch-Johnsen K, Thorsteinsson B. Frequency and risk factors of severe hypoglycemia in insulin-treated type 2 diabetes: a literature survey. J Diabetes Complicat. 2006;20:402–8. https://doi.org/10.1016/j.jdiacomp.2005.08.005.
Carlson AL, Daniel TD, DeSantis A, et al. Flash glucose monitoring in type 2 diabetes managed with basal insulin in the USA: a retrospective real-world chart review study and meta-analysis. BMJ Open Diabetes Res Care. 2022;10:e002590. https://doi.org/10.1136/bmjdrc-2021-002590.
Miller E, Kerr MSD, Roberts GJ, Nabutovsky Y, Wright E. Flash CGM associated with event reduction in nonintensive diabetes therapy. Am J Manag Care. 2021;27:372–7. https://doi.org/10.37765/ajmc.2021.88780.
Ziegler R, Heinemann L, Freckmann G, Schnell O, Hinzmann R, Kulzer B. Intermittent use of continuous glucose monitoring: expanding the clinical value of CGM. J Diabetes Sci Technol. 2021;15:684–94. https://doi.org/10.1177/1932296820905577.
Taylor PJ, Thompson CH, Brinkworth GD. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: a narrative review. J Diabetes Invest. 2018;9:713–25. https://doi.org/10.1111/jdi.12807.
Price DA, Deng Q, Kipnes M, Beck SE. Episodic real-time CGM use in adults with type 2 diabetes: results of a pilot randomized controlled trial. Diabetes Ther. 2021;12:2089–99. https://doi.org/10.1007/s13300-021-01086-y.
Moon SJ, Kim K, Lee WJ, Lee MY, Vigersky R, Park C. Efficacy of intermittent short-term use of a real-time continuous glucose monitoring system in non-insulin-treated patients with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2022. https://doi.org/10.1111/dom.14852.
Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract. 2008;80:371–9. https://doi.org/10.1016/j.diabres.2008.01.006.
Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH. Hypoglycemia in type 2 diabetes—more common than you think. J Diabetes Sci Technol. 2015;9:999–1005. https://doi.org/10.1177/1932296815581052.
Choudhary P, Portu S, Delbaere A, Lyon J, Pickup JC. A modelling study of the budget impact of improved glycaemic control in adults with type 1 diabetes in the UK. Diabetic Med. 2019;36:988–94. https://doi.org/10.1111/dme.13924.
Rotondi MA, Wong O, Riddell M, Perkins B. Population-level impact and cost-effectiveness of continuous glucose monitoring and intermittently scanned continuous glucose monitoring technologies for adults with type 1 diabetes in Canada: a modeling study. Diabetes Care. 2022. https://doi.org/10.2337/dc21-2341.
Roze S, Isitt JJ, Smith-Palmer J, et al. Long-term cost-effectiveness the Dexcom G6 real-time continuous glucose monitoring system compared with self-monitoring of blood glucose in people with type 1 diabetes in France. Diabetes Ther. 2021;12:235–46. https://doi.org/10.1007/s13300-020-00959-y.
Roze S, Isitt J, Smith-Palmer J, Javanbakht M, Lynch P. Long-term cost-effectiveness of Dexcom G6 real-time continuous glucose monitoring versus self-monitoring of blood glucose in patients with type 1 diabetes in the UK. Diabetes Care. 2020;43:2411–7. https://doi.org/10.2337/dc19-2213.
Fonda SJ, Graham C, Munakata J, Powers JM, Price D, Vigersky RA. The cost-effectiveness of real-time continuous glucose monitoring (RT-CGM) in type 2 diabetes. J Diabetes Sci Technol. 2016;10:898–904. https://doi.org/10.1177/1932296816628547.
Sierra JA, Shah M, Gill MS, et al. Clinical and economic benefits of professional CGM among people with type 2 diabetes in the United States: analysis of claims and lab data. J Med Econ. 2018;21:225–30. https://doi.org/10.1080/13696998.2017.1390474.
Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019. https://doi.org/10.2337/dc18-0166.
Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–9. https://doi.org/10.1016/s2213-8587(18)30051-2.
Hall H, Perelman D, Breschi A, et al. Glucotypes reveal new patterns of glucose dysregulation. Plos Biol. 2018;16:e2005143. https://doi.org/10.1371/journal.pbio.2005143.
Safford MM, Shewchuk R, Qu H, et al. Reasons for not intensifying medications: differentiating “clinical inertia” from appropriate care. J Gen Intern Med. 2007;22:1648–55. https://doi.org/10.1007/s11606-007-0433-8.
Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia in intensifying therapy among people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016. https://doi.org/10.1111/dom.12626.
Seidu S, Than T, Kar D, et al. Therapeutic inertia amongst general practitioners with interest in diabetes. Prim Care Diabetes. 2018;12:87–91. https://doi.org/10.1016/j.pcd.2017.09.001.
Doyle-Delgado K, Chamberlain JJ, Shubrook JH, Skolnik N, Trujillo J. Pharmacologic approaches to glycemic treatment of type 2 diabetes: synopsis of the 2020 American Diabetes Association’s standards of medical care in diabetes clinical guideline. Ann Intern Med. 2020;173:813–21. https://doi.org/10.7326/m20-2470.
Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427–37. https://doi.org/10.1111/dom.13088.
Kaewbut P, Kosachunhanun N, Phrommintikul A, Chinwong D, Hall JJ, Chinwong S. Time to treatment intensification to reduce diabetes-related complications: a post hoc study. Healthcare (Basel). 2022;10:1673. https://doi.org/10.3390/healthcare10091673.
Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. https://doi.org/10.1186/s12933-015-0260-x.
Bain SC, Hansen BB, Hunt B, Chubb B, Valentine WJ. Evaluating the burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the UK. J Med Econ. 2019;23:1–1. https://doi.org/10.1080/13696998.2019.1645018.
Tsotra F, Kappel M, Peristeris P, et al. The societal impact of early intensified treatment in patients with type 2 diabetes mellitus. J Comp Effect Res. 2022. https://doi.org/10.2217/cer-2022-0110.
Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes. Diabetes Care. 2005;28:2543–5. https://doi.org/10.2337/diacare.28.10.2543.
Harris S, Levrat-Guillen F. Use of the FreeStyle Libre® system in diabetes treatment for people with T2D: results from a retrospective cohort study using Canadian private payer claims database. Diabetes Obesity Metab. 2023:25(6):1704–13.
Simonson GD, Bergenstal RM, Johnson ML, Davidson JL, Martens TW. Effect of professional CGM (pCGM) on glucose management in type 2 diabetes patients in primary care. J Diabetes Sci Technol. 2021;15:539–45. https://doi.org/10.1177/1932296821998724.
Sherrill CH, Houpt CT, Dixon EM, Richter SJ. Effect of pharmacist-driven professional continuous glucose monitoring in adults with uncontrolled diabetes. J Manag Care Spec. 2020;26:600–9. https://doi.org/10.18553/jmcp.2020.26.5.600.
Wallace T, Heath J, Koebbel C. The impact of flash glucose monitoring on adults with type 1 diabetes’ eating habits and relationship with food. Diabetes Res Clin Pract. 2023;196:110230. https://doi.org/10.1016/j.diabres.2022.110230.
Polonsky WH, Fortmann AL, Soriano EC, Guzman SJ, Funnell MM. The AH-HA! project: transforming group diabetes self-management education through the addition of flash glucose monitoring. Diabetes Technol Ther. 2022. https://doi.org/10.1089/dia.2022.0419.
Rivera-Ávila DA, Esquivel-Lu AI, Salazar-Lozano CR, Jones K, Doubova SV. The effects of professional continuous glucose monitoring as an adjuvant educational tool for improving glycemic control in patients with type 2 diabetes. BMC Endocr Disord. 2021;21:79. https://doi.org/10.1186/s12902-021-00742-5.
Oser TK, Litchman ML, Allen NA, et al. Personal continuous glucose monitoring use among adults with type 2 diabetes: clinical efficacy and economic impacts. Curr Diabetes Rep. 2021;21:49. https://doi.org/10.1007/s11892-021-01408-1.
Bergenstal RM, Kerr MSD, Roberts GJ, Souto D, Nabutovsky Y, Hirsch IB. Flash CGM is associated with reduced diabetes events and hospitalizations in insulin-treated type 2 diabetes. J Endocr Soc. 2021;5:13. https://doi.org/10.1210/jendso/bvab013.
FreeStyle Libre 2 Flash Glucose Monitoring System. Haute Autorite Sante 2020.
Miao R, Wei W, Baser O, Xie L. Real world outcomes of adding rapid-acting insulin versus switching to analog premix insulin among US patients with type 2 diabetes treated with insulin glargine. Patient Prefer Adher. 2013;7:951–60. https://doi.org/10.2147/ppa.s49287.
Roussel R, Detournay B, Boultif Z, Bahloul A, Teissier C, Charbonnel B. Persistence with basal insulin and frequency of hypoglycemia requiring hospitalization in patients with type 2 diabetes. Diabetes Ther. 2020;11:1861–72. https://doi.org/10.1007/s13300-020-00874-2.
Idris I, Gulati K, Perez-Nieves M, et al. Associated factors that influenced persistence with basal analog insulin therapy among people with type 2 diabetes: an exploratory analysis from a UK real-world sample. Prim Care Diabetes. 2019;13:106–12. https://doi.org/10.1016/j.pcd.2018.09.002.
Aleppo G, Beck RW, Bailey R, et al. The effect of discontinuing continuous glucose monitoring in adults with type 2 diabetes treated with basal insulin. Diabetes Care. 2021;44:2729–37. https://doi.org/10.2337/dc21-1304.
Elliott T, Beca S, Beharry R, Tsoukas MA, Zarruk A, Abitbol A. The impact of flash glucose monitoring on glycated hemoglobin in type 2 diabetes managed with basal insulin in Canada: a retrospective real-world chart review study. Diabetes Vasc Dis Re. 2021;18:14791641211021374. https://doi.org/10.1177/14791641211021374.
Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32:1626–31. https://doi.org/10.2337/dc09-0586.
Alves C, Della-Manna T, Albuquerque CTM. Cystic fibrosis-related diabetes: an update on pathophysiology, diagnosis, and treatment. J Pediatr Endocrinol Metab. 2020;33:835–43. https://doi.org/10.1515/jpem-2019-0484.
Pozo L, Bello F, Mendez Y, Surani S. Cystic fibrosis-related diabetes: the unmet need. World J Diabetes. 2020;11:213–7. https://doi.org/10.4239/wjd.v11.i6.213.
Chamnan P, Shine BSF, Haworth CS, Bilton D, Adler AI. Diabetes as a determinant of mortality in cystic fibrosis. Diabetes Care. 2010;33:311–6. https://doi.org/10.2337/dc09-1215.
Kumar S, Pallin M, Soldatos G, Teede H. Comparison of continuous glucose monitoring to reference standard oral glucose tolerance test for the detection of dysglycemia in cystic fibrosis: a systematic review. J Clin Transl Endocrinol. 2022;30:100305. https://doi.org/10.1016/j.jcte.2022.100305.
Zorron M, Marson FAL, Morcillo AM, et al. Can continuous glucose monitoring predict cystic fibrosis-related diabetes and worse clinical outcome? J Bras Pneumol 2022;48:202. https://doi.org/10.36416/1806-3756/e20210307.
Scully KJ, Sherwood JS, Martin K, et al. Continuous glucose monitoring and HbA1c in cystic fibrosis: clinical correlations and implications for CFRD diagnosis. J Clin Endocrinol Metab. 2021;107:e1444–54. https://doi.org/10.1210/clinem/dgab857.
Kumar S, Soldatos G, Ranasinha S, Teede H, Pallin M. Continuous glucose monitoring versus self-monitoring of blood glucose in the management of cystic fibrosis related diabetes: a systematic review and meta-analysis. J Cyst Fibros. 2022. https://doi.org/10.1016/j.jcf.2022.07.013.
Gojsina B, Minic P, Todorovic S, Soldatovic I, Sovtic A. Continuous glucose monitoring as a valuable tool in the early detection of diabetes related to cystic fibrosis. Front Pediatr. 2021;9: 659728. https://doi.org/10.3389/fped.2021.659728.
UK Cystic Fibrosis Registry. 2021 Annual Data Report. https://www.cysticfibrosis.org.uk/sites/default/files/2022-10/CFT_2021-Annual-Data-Report-WEB.pdf, 1.24 CF-related diabetes, 34.
Grace T, Salyer J. Use of real-time continuous glucose monitoring improves glycemic control and other clinical outcomes in type 2 diabetes patients treated with less intensive therapy. Diabetes Technol Ther. 2022;24:26–31. https://doi.org/10.1089/dia.2021.0212.
Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. https://doi.org/10.1016/j.diabres.2018.02.023.
Bao S, Bailey R, Calhoun P, Beck RW. Effectiveness of continuous glucose monitoring in older adults with type 2 diabetes treated with basal insulin. Diabetes Technol Ther. 2022;24:299–306. https://doi.org/10.1089/dia.2021.0494.
Guerci B, Levrat-Guillen F, Vicaut E, et al. Reduced acute diabetes events after FreeStyle Libre® system initiation in people 65 years or older with type 2 diabetes on intensive insulin therapy in France. Diabet Technol Ther. 2023. https://doi.org/10.1089/dia.2023.0034.
Munshi MN, Segal AR, Suhl E, et al. Frequent hypoglycemia among elderly patients with poor glycemic control. Arch Intern Med. 2011;171:362–4. https://doi.org/10.1001/archinternmed.2010.539.
Lipska KJ, Warton EM, Huang ES, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes. Diabetes Care. 2013;36:3535–42. https://doi.org/10.2337/dc13-0610.
Meneilly GS, Tessier D. Diabetes in the elderly. Diabetic Med. 1995;12:949–60. https://doi.org/10.1111/j.1464-5491.1995.tb00405.x.
Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol Ser. 2001;56:M5-13. https://doi.org/10.1093/gerona/56.1.m5.
Schütt M, Fach E-M, Seufert J, et al. Multiple complications and frequent severe hypoglycaemia in ‘elderly’ and ‘old’ patients with type 1 diabetes. Diabetic Med. 2012;29:e176–9. https://doi.org/10.1111/j.1464-5491.2012.03681.x.
Wallander M, Axelsson KF, Nilsson AG, Lundh D, Lorentzon M. Type 2 diabetes and risk of hip fractures and non-skeletal fall injuries in the elderly: a study from the fractures and fall injuries in the elderly cohort (FRAILCO). J Bone Miner Res. 2017;32:449–60. https://doi.org/10.1002/jbmr.3002.
Mattishent K, Richardson K, Dhatariya K, Savva GM, Fox C, Loke YK. The effects of hypoglycaemia and dementia on cardiovascular events, falls and fractures and all-cause mortality in older individuals: a retrospective cohort study. Diabetes Obes Metab. 2019;21:2076–85. https://doi.org/10.1111/dom.13769.
Mattishent K, Loke YK. Is avoidance of hypoglycaemia a better target than HbA1C in older people with diabetes? Brit J Clin Pharmaco. 2021;87:9–11. https://doi.org/10.1111/bcp.14517.
Kosjerina V, Carstensen B, Jørgensen ME, et al. Discontinuation of diabetes medication in the 10 years before death in Denmark: a register-based study. Lancet Heal Longev. 2021;2:e561–70. https://doi.org/10.1016/s2666-7568(21)00170-7.
Hart HE, Ditzel K, Rutten GE, et al. De-intensification of blood glucose lowering medication in people identified as being over-treated: a mixed methods study. Patient Prefer Adher. 2019;13:1775–83. https://doi.org/10.2147/ppa.s208947.
Selvin E, Wang D, Tang O, Minotti M, Echouffo-Tcheugui JB, Coresh J. Glucose patterns in very old adults: a pilot study in a community-based population. Diabetes Technol Ther. 2021;23:737–44. https://doi.org/10.1089/dia.2021.0156.
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia. 2011;7:280–92. https://doi.org/10.1016/j.jalz.2011.03.003.
Athanasaki A, Melanis K, Tsantzali I, et al. Type 2 diabetes mellitus as a risk factor for Alzheimer’s disease: review and meta-analysis. Biomed. 2022;10:778. https://doi.org/10.3390/biomedicines10040778.
Sinclair A, Abdelhafiz A. Cognitive dysfunction in older adults with type 2 diabetes: links, risks, and clinical implications. Clin Geriatr Med. 2020;36:407–17. https://doi.org/10.1016/j.cger.2020.04.002.
Bordier L, Doucet J, Boudet J, Bauduceau B. Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes Metab. 2014;40:331–7. https://doi.org/10.1016/j.diabet.2014.02.002.
Laiteerapong N, Karter AJ, Liu JY, et al. Correlates of quality of life in older adults with diabetes. Diabetes Care. 2011;34:1749–53. https://doi.org/10.2337/dc10-2424.
Angeli F, Reboldi G, Poltronieri C, et al. Hyperglycemia in acute coronary syndromes: from mechanisms to prognostic implications. Ther Adv Cardiovasc Dis. 2015;9:412–24. https://doi.org/10.1177/1753944715594528.
Vedantam D, Poman DS, Motwani L, Asif N, Patel A, Anne KK. Stress-induced hyperglycemia: consequences and management. Cureus. 2022;14:e26714. https://doi.org/10.7759/cureus.26714.
Marfella R, Federici M, Paolisso G. Editorial: hyperglycemia and coronary artery diseases: physio-pathological findings and therapeutic implications. Front Pharmacol. 2022;13: 901815. https://doi.org/10.3389/fphar.2022.901815.
Malmberg K, Rydén L, Hamstent A, Herlitz J, Waldenström A, Wedel H. Effects of insulin treatment on cause-specific one-year mortality and morbidity in diabetic patients with acute myocardial infarction. Eur Heart J. 1996;17:1337–44. https://doi.org/10.1093/oxfordjournals.eurheartj.a015067.
Ritsinger V, Malmberg K, Mårtensson A, Rydén L, Wedel H, Norhammar A. Intensified insulin-based glycaemic control after myocardial infarction: mortality during 20 year follow-up of the randomised Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI 1) trial. Lancet Diabetes Endocrinol. 2014;2:627–33. https://doi.org/10.1016/s2213-8587(14)70088-9.
Malmberg K, Rydén L, Wedel H, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650–61. https://doi.org/10.1093/eurheartj/ehi199.
Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:4533. https://doi.org/10.1136/bmj.f4533.
Kissela BM, Khoury J, Kleindorfer D, et al. Epidemiology of ischemic stroke in patients with diabetes. Diabetes Care. 2005;28:355–9. https://doi.org/10.2337/diacare.28.2.355.
Bruno A, Levine SR, Frankel MR, et al. Admission glucose level and clinical outcomes in the NINDS rt-PA stroke trial. Neurology. 2002;59:669–74. https://doi.org/10.1212/wnl.59.5.669.
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients. Stroke. 2001;32:2426–32. https://doi.org/10.1161/hs1001.096194.
Krinock MJ, Singhal NS. Diabetes, stroke, and neuroresilience: looking beyond hyperglycemia. Ann N Y Acad Sci. 2021;1495:78–98. https://doi.org/10.1111/nyas.14583.
Rodgers ML, Fox E, Abdelhak T, et al. Care of the patient with acute ischemic stroke (Endovascular/Intensive Care Unit-Postinterventional Therapy): update to 2009 comprehensive nursing care scientific statement: a scientific statement from the American Heart Association. Stroke. 2021;52:e198-210. https://doi.org/10.1161/str.0000000000000358.
Yim C, Mansell K, Hussein N, Arnason T. Current cancer therapies and their influence on glucose control. World J Diabetes. 2021;12:1010–25. https://doi.org/10.4239/wjd.v12.i7.1010.
Gerards MC, van der Velden DL, Baars JW, et al. Impact of hyperglycemia on the efficacy of chemotherapy—a systematic review of preclinical studies. Crit Rev Oncol Hemat. 2017;113:235–41. https://doi.org/10.1016/j.critrevonc.2017.03.007.
Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249–55. https://doi.org/10.1097/ccm.0b013e318181039a.
Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, DeVries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838–42. https://doi.org/10.1097/ccm.0b013e3181cc4be9.
Yang J, Jia B, Qiao Y, Chen W, Qi X. Variations of blood glucose in cancer patients during chemotherapy. Niger J Clin Pract. 2016;19:704. https://doi.org/10.4103/1119-3077.187323.
Latham R, Lancaster AD, Covington JF, Pirolo JS, Thomas CS. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607–12. https://doi.org/10.1086/501830.
Martin ET, Kaye KS, Knott C, et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37:88–99. https://doi.org/10.1017/ice.2015.249.
Buehler L, Fayfman M, Alexopoulos A-S, et al. The impact of hyperglycemia and obesity on hospitalization costs and clinical outcome in general surgery patients. J Diabetes Complicat. 2015;29:1177–82. https://doi.org/10.1016/j.jdiacomp.2015.07.027.
Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C, DIAMOND Study Group. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. 2017;11:1138–46. https://doi.org/10.1177/1932296817704445.
Gomes V, Ferreira F, Guerra J, Bugalho MJ. New-onset diabetes after kidney transplantation: incidence and associated factors. World J Diabetes. 2018;9:132–7. https://doi.org/10.4239/wjd.v9.i7.132.
Vest AR, Cherikh WS, Noreen SM, Stehlik J, Khush KK. New-onset diabetes mellitus after adult heart transplantation and the risk of renal dysfunction or mortality. Transplantation. 2022;106:178–87. https://doi.org/10.1097/tp.0000000000003647.
Davidson J, Wilkinson A, Dantal J, et al. New-onset diabetes after transplantation: 2003 international consensus guidelines. Transplantation. 2003;75:3–24. https://doi.org/10.1097/01.tp.0000069952.49242.3e.
Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV impact of post-transplant diabetes. Kidney Int. 2002;62:1440–6. https://doi.org/10.1111/j.1523-1755.2002.kid582.x.
Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2018;42: 181444. https://doi.org/10.2337/dc18-1444.
Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41: 181131. https://doi.org/10.2337/dc18-1131.
Sobngwi E, Ashuntantang G, Ndounia E, et al. Continuous interstitial glucose monitoring in non-diabetic subjects with end-stage renal disease undergoing maintenance haemodialysis. Diabetes Res Clin Pract. 2010;90:22–5. https://doi.org/10.1016/j.diabres.2010.06.001.
Joubert M, Fourmy C, Henri P, Ficheux M, Lobbedez T, Reznik Y. Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract. 2015;107:348–54. https://doi.org/10.1016/j.diabres.2015.01.026.
Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41:181581. https://doi.org/10.2337/dc18-1581.
Galindo RJ, Beck RW, Scioscia MF, Umpierrez GE, Tuttle KR. Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev. 2020;41:756–74. https://doi.org/10.1210/endrev/bnaa017.
Gomez-Peralta F, Choudhary P, Cosson E, Irace C, Rami-Merhar B, Seibold A. Understanding the clinical implications of differences between GMI and HbA1c. Diabetes Obes Metab. 2022. https://doi.org/10.1111/dom.14638.
Murphy HR, Howgate C, O’Keefe J, et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 2021;9:153–64. https://doi.org/10.1016/s2213-8587(20)30406-x.
Evers IM, de Valk HW, Visser GHA. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ. 2004;328:915. https://doi.org/10.1136/bmj.38043.583160.ee.
Dirar AM, Doupis J. Gestational diabetes from A to Z. World J Diabetes. 2017;8:489–511. https://doi.org/10.4239/wjd.v8.i12.489.
Shen S-Y, Žurauskienė J, Wei D-M, et al. Identification of maternal continuous glucose monitoring metrics related to newborn birth weight in pregnant women with gestational diabetes. Endocrine. 2021;74:290–9. https://doi.org/10.1007/s12020-021-02787-x.
Law GR, Alnaji A, Alrefaii L, et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care. 2019;42:810–5. https://doi.org/10.2337/dc18-2212.
Gáborová M, Doničová V, Bačová I, et al. Glycaemic variability and risk factors of pregnant women with and without gestational diabetes mellitus measured by continuous glucose monitoring. Int J Environ Res Public Health. 2021;18:3402. https://doi.org/10.3390/ijerph18073402.
Majewska A, Stanirowski PJ, Wielgoś M, Bomba-Opoń D. Efficacy of continuous glucose monitoring on glycaemic control in pregnant women with gestational diabetes mellitus—a systematic review. J Clin Med. 2022;11:2932. https://doi.org/10.3390/jcm11102932.
García-Moreno RM, Benítez-Valderrama P, Barquiel B, et al. Efficacy of continuous glucose monitoring on maternal and neonatal outcomes in gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Diabet Med. 2022;39:e14703. https://doi.org/10.1111/dme.14703.
Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165:849–57. https://doi.org/10.1093/aje/kwk071.
Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in early childhood and risk of sustained obesity. New Engl J Med. 2018;379:1303–12. https://doi.org/10.1056/nejmoa1803527.
Oxman R, Roe AH, Jagdeesh U, Putman MS. Gestational and pregestational diabetes in pregnant women with cystic fibrosis. J Clin Transl Endocrinol. 2022;27: 100289. https://doi.org/10.1016/j.jcte.2021.100289.
Ashcroft A, Chapman S, Mackillop L. The outcome of pregnancy in women with cystic fibrosis: a UK population-based descriptive study. Bjog Int J Obstetrics Gynaecol. 2020;127:1696–703. https://doi.org/10.1111/1471-0528.16423.
Murphy HR, Rayman G, Duffield K, et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care. 2007;30:2785–91. https://doi.org/10.2337/dc07-0500.
Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390:2347–59. https://doi.org/10.1016/s0140-6736(17)32400-5.
Scott EM, Murphy HR, Kristensen KH, et al. Continuous glucose monitoring metrics and birth weight: informing management of type 1 diabetes throughout pregnancy. Diabetes Care. 2022;45:1724–34. https://doi.org/10.2337/dc22-0078.
Kristensen K, Ögge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62:1143–53. https://doi.org/10.1007/s00125-019-4850-0.
Bartal MF, Cornthwaite JA, Ghafir D, et al. Time in range and pregnancy outcomes in people with diabetes using continuous glucose monitoring. Am J Perinat. 2022. https://doi.org/10.1055/a-1904-9279.
Dori-Dayan N, Cukierman-Yaffe T, Kedar N, et al. Maternal glucose variability during pregnancy & birthweight percentile in women with pre-gestational diabetes. Gynecol Endocrinol. 2021;37:1116–20. https://doi.org/10.1080/09513590.2021.1993814.
Lemaitre M, Faiz K, Baudoux F, Subtil D, Vambergue A. Intermittently scanned continuous glucose monitoring is associated with lower spontaneous abortion rate compared with conventional blood glucose monitoring in pregnant women with type 1 diabetes: an observational study. Diabetes Vasc Dis Res. 2022;19:14791641221136836. https://doi.org/10.1177/14791641221136837.
Majewska A, Stanirowski P, Wielgoś M, Bomba-Opoń D. Flash glucose monitoring in gestational diabetes mellitus: study protocol for a randomised controlled trial. BMJ Open. 2021;11:e041486. https://doi.org/10.1136/bmjopen-2020-041486.
Choudhary P, Bellido V, Graner M, et al. The challenge of sustainable access to telemonitoring tools for people with diabetes in Europe: lessons from COVID-19 and beyond. Diabetes Ther. 2021;2:1–17. https://doi.org/10.1007/s13300-021-01132-9.
Commission E. Benchmarking deployment of eHealth among general practitioners. 2018.
Danne T, Limbert C, Domingo MP, et al. Telemonitoring, telemedicine and time in range during the pandemic: paradigm change for diabetes risk management in the post-COVID future. Diabetes Ther. 2021;12:2289–310. https://doi.org/10.1007/s13300-021-01114-x.
Silva-Tinoco R, de la Torre-Saldaña V. The imperious need for telemedicine for the care of diabetes during the COVID-19 pandemic. A comprehensive approach study. Gac Med Mex. 2021;157:309–12. https://doi.org/10.24875/gmm.m21000563.
Pogorzelska K, Marcinowicz L, Chlabicz S. A qualitative study of primary care physicians’ experiences with telemedicine during the COVID-19 pandemic in North-Eastern Poland. Int J Environ Res Public Health. 2023;20:1963. https://doi.org/10.3390/ijerph20031963.
Poitras M-E, Poirier M-D, Couturier Y, et al. Chronic conditions patient’s perception of post-COVID-19 pandemic teleconsulting continuation in primary care clinics: a qualitative descriptive study. BMJ Open. 2022;12:e066871. https://doi.org/10.1136/bmjopen-2022-066871.
Acknowledgements
Funding
The Rapid Service Fee was provided by Abbott Diabetes Care, who also provided support for the medical writer.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
Editorial assistance in the preparation of this manuscript was provided by Dr Robert Brines of Bite Medical Consulting. Funding for writing support was provided by Abbott Diabetes Care.
Author Contributions
Tomasz Klupa, Leszek Czupryniak, Grzegorz Dzida, Piotr Fichna, Przemyslawa Jarosz-Chobot, Janusz Gumprecht, Malgorzata Mysliwiec, Agnieszka Szadkowska, Dorota Bomba-Opon, Krzysztof Czajkowski, Maciej T. Malecki and Dorota Zozulinska-Ziolkiewicz discussed the topics contained in this manuscript over two expert advisory panels. All authors contributed to the concept and design of the manuscript and worked collaboratively to review and prepare the final manuscript.
Disclosures
Tomasz Klupa has received honoraria from Abbott, Ascencia, Medtronic, Roche, and research support from Medtronic. Leszek Czupryniak has received honoraria from Abbott, Abbott, Ascencia, Medtronic, Roche. Grzegorz Dzida has received honoraria from Abbott, Roche. Piotr Fichna has received honoraria from Abbott. Przemyslawa Jarosz-Chobot has received honoraria from Abbott, Ascencia, Dexcom, Medtronic, Roche. Janusz Gumprecht has received honoraria from Abbott, Ascencia. Malgorzata Mysliwiec has received honoraria from Abbott, Ascencia, Dexcom, Medtronic. Agnieszka Szadkowska has received honoraria from Abbott, Ascencia, Dexcom, Medtronic. Dorota Bomba-Opon has received honoraria from Abbott. Krzysztof Czajkowski has received honoraria from Abbott. Maciej T. Malecki has received honoraria from Abbott, Ascensia, Dexcom, Medtronic, Roche. Dorota Zozulinska-Ziolkiewicz has received honoraria from Abbott, Ascensia, Dexcom, Medtronic, Roche.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. All statements and recommendations comply with the 1964 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Klupa, T., Czupryniak, L., Dzida, G. et al. Expanding the Role of Continuous Glucose Monitoring in Modern Diabetes Care Beyond Type 1 Disease. Diabetes Ther 14, 1241–1266 (2023). https://doi.org/10.1007/s13300-023-01431-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01431-3