Abstract
Introduction
Individuals with type 2 diabetes (T2D) are at high risk of experiencing atherosclerotic cardiovascular disease (ASCVD), which is associated with morbidity, mortality and healthcare resource utilisation. Clinical guidelines recommend the use of glucose-lowering medications with cardiovascular benefits in individuals with T2D and cardiovascular disease, but there is evidence that this is not reflected in clinical practice. We used linked national registry data from Sweden to compare outcomes in people with T2D and ASCVD against matched controls with T2D without ASCVD, over 5 years. Direct costs (inpatient, outpatient and selected drug costs), indirect costs resulting from work absence, early retirement, cardiovascular events and mortality were examined.
Methods
Individuals with T2D who were at least 16 years old and were alive and resident in Sweden on 1 January 2012 were identified in an existing database. In four separate analyses, individuals with a record indicating ASCVD according to a broad definition, peripheral artery disease (PAD), stroke or myocardial infarction (MI) before 1 January 2012 were identified using diagnosis and/or procedure codes and propensity score matched 1:1 to controls with T2D and without ASCVD, using covariates for birth, sex and level of education in 2012. Follow-up continued until death, migration from Sweden or the end of the study period in 2016.
Results
In total, 80,305 individuals with ASCVD, 15,397 individuals with PAD, 17,539 individuals with previous stroke and 25,729 individuals with previous MI were included. Total mean annual costs per person were €14,785 for PAD (2.7 × costs for controls), €11,397 for previous stroke (2.2 × controls), €10,730 for ASCVD (1.9 × controls) and €10,342 for previous MI (1.7 × controls). Indirect costs and costs of inpatient care were the major cost drivers. ASCVD, PAD, stroke and MI were all associated with an increased likelihood of early retirement, cardiovascular events and mortality.
Conclusions
ASCVD is associated with considerable costs, morbidity and mortality in individuals with T2D. These results support structured assessment of ASCVD risk and broader implementation of guideline-recommended treatments in T2D healthcare.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality in diabetes, and is associated with high healthcare resource utilisation, but few studies assess the impact of this on both healthcare systems and society |
We compared direct and indirect costs, early retirement, cardiovascular events and mortality for individuals with type 2 diabetes (T2D) and ASCVD, peripheral artery disease, previous stroke or previous myocardial infarction, and matched controls with T2D without ASCVD |
What was learned from the study? |
Total costs were nearly double in individuals with ASCVD compared with matched controls; indirect costs from work absence and inpatient costs were the main cost drivers |
ASCVD was associated with early retirement, stroke, myocardial infarction and mortality |
These results support structured assessment of ASCVD risk and broader implementation of guideline-recommended treatments in T2D healthcare |
Introduction
There is evidence that nearly one-third of individuals with type 2 diabetes (T2D) have established cardiovascular disease (CVD) [1], and a similar proportion have atherosclerotic cardiovascular disease (ASCVD) [2], which manifests as sometimes fatal cardiovascular conditions and events, including transient ischaemic attacks, stroke, myocardial infarction (MI) and peripheral artery disease (PAD) [3]. People with T2D are more likely to experience ASCVD events than those without T2D [4, 5], and these events are likely to happen earlier in life and to be more severe [6, 7]. Despite improvements in cardiovascular risk factor control and reductions in diabetes-related mortality over the past decades, excess all-cause and CVD mortality in diabetes persist [8], and ASCVD is the leading cause of both morbidity and mortality in diabetes [9]. Clinical guidelines for the management of diabetes and cardiovascular disease [10, 11], including the 2022 consensus report by the American Diabetes Association and the European Association for the Study of Diabetes [12], recommend the use of a glucagon-like peptide 1 receptor agonist (GLP-1 RA) or a sodium-glucose-like cotransporter 2 inhibitor (SGLT2i) in people with T2D and CVD or high CVD risk. However, data from the CAPTURE study, which included nearly 10,000 adults with T2D treated in primary or specialist care in 13 countries, indicate that these medications are prescribed with equal frequency in individuals with and without CVD, suggesting that T2D is often not managed according to guidelines [13]. The 2021 annual report from the Swedish national quality registry SWEDEHEART showed that only 43% of people with T2D who experienced an acute MI had been prescribed a GLP-1 RA and/or an SGLT2i in the 6–10 weeks following this event, with considerable variation between treatment centres [14].
The clinical impact of ASCVD in T2D is highlighted by its link to healthcare resource utilisation. US data from 2015 showed that ASCVD in individuals with T2D is associated with an increased likelihood of inpatient admissions, outpatient visits and emergency room visits, alongside increased healthcare costs [15]. ASCVD is associated both with high direct healthcare costs, owing to the need for acute treatment and long-term medical management, and high indirect costs, resulting from work absence and lost productivity [16,17,18]. However, relatively few studies of CVD in T2D report indirect costs [19].
Population-based, longitudinal, linked national health registries from Nordic countries are widely used in such analyses examining the disease course of chronic conditions, owing to the breadth of data available. Recent Danish studies have investigated the risk of PAD in individuals with T2D and coronary artery disease [20, 21], and the costs of T2D-related complications [22]. Pooled registry data from Sweden and Norway have also been used to compare morbidity in type 1 diabetes and T2D [23], and a database linking Swedish national administrative registers has been used to assess the impact of complications of T2D on productivity [24] and the costs of hospital-based care [25].
In this study, we used the national longitudinal data from earlier publications [24, 25] to assess the effects of ASCVD on direct and indirect costs, early retirement, work absence, cardiovascular events and mortality in individuals with T2D, over a 5-year period. We compared these outcomes for cases against matched controls with T2D without ASCVD, and also assessed the trajectory of direct costs before and after the occurrence of ASCVD. In addition to identifying individuals with ASCVD according to a broad definition, we conducted analyses in subgroups of individuals with PAD, previous stroke and previous MI.
Methods
Study Design and Data Sources
This observational, retrospective, closed-cohort study used data from an existing database at the Swedish Institute for Health Economics. The database, full details of which have been reported by Persson et al. [24], cross-links individual patient data from the National Board of Health and Welfare in Sweden, Statistics Sweden and Försäkringskassan (the Swedish Social Insurance Agency), spanning the period 1997–2017. Together, these data sources contain information on healthcare resource utilisation, demographic and socioeconomic data, and work absence (Table S1 in the Supplementary Material).
Study Population
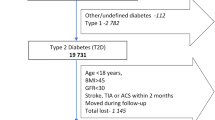
Individuals with T2D who were at least 16 years old and alive and resident in Sweden on 1 January 2012 (index date) were eligible for inclusion in the study. T2D was identified as described in the Methods in the Supplementary Material. Individuals with a record of ASCVD, PAD, stroke or MI in the National Patient Register before the index date (1997–2011) were then identified according to the presence of relevant International Classification of Diseases, tenth revision (ICD-10) codes and procedure codes (Table 1).
Individuals with T2D with a record of ASCVD, PAD, stroke or MI were propensity score matched [26, 27] 1:1 to controls with T2D but no record of ASCVD before 1 January 2012, using logistic regression with nearest neighbour matching, with covariates for birth, sex and level of education in 2012. Level of education was considered to be a proxy for socioeconomic status. Individuals could be included in more than one of the ASCVD, PAD, stroke and MI groups.
Outcomes
Outcomes during 2012–2016 (the study period) were compared between individuals with T2D and each condition and their matched controls. Follow-up for each individual was until the end of the study period in 2016, death or migration, whichever was earliest. Direct costs, comprising inpatient costs, outpatient costs and the costs of selected drugs, and indirect costs, resulting from work absence, were estimated as described in the Methods in the Supplementary Material and summed to give total mean annual costs. Any indirect costs for individuals aged 66 years or older were not accounted for. Codes for hospital-based care and drug costs are shown in Tables S2 and S3 in the Supplementary Material. Both mean annual costs per person and 5-year cumulative costs per person are presented. Mean cumulative costs were calculated among individuals alive in each separate year. All costs in Swedish krona were converted to euros using the average exchange rate for 2016. The numbers of days absent from work per year were also compared in working age individuals, who were defined as individuals aged younger than 66 years.
The risks of entering full-time early retirement (365 days of sickness and activity compensation in the year from Försäkringskassan before the age of 66 years), or experiencing stroke, MI or death during the study period were compared between groups, using Cox proportional regression adjusted for year of birth, sex, history of comorbidities, level of education and marital status, as described in the Methods in the Supplementary Material. Stroke or MI as outcome variables during the study period were defined as the first inpatient admission with the condition as main diagnosis.
Continuous variables are reported as mean and standard error of the mean (SEM), and categorical variables are reported as actual numbers and proportions (%).
Incident Analyses
To assess the impact of ASCVD, PAD, stroke or MI in the year that it occurred, subsets of individuals with their first documented occurrence of each condition in 2011 were identified. Direct costs for each year from 2007 to 2016 were compared between these individuals and the controls with no record of ASCVD before 1 January 2012. Data were also summarised to show observed events during the study period with transitions between four selected states: no (new) events, observed stroke in study year, observed MI in study year, both MI and stroke in study year, and death in study year. New events were defined as events occurring for the first time during the study period. Recurrent stroke and MI events occurring during the study period (i.e. the second occurrence of stroke or MI between 2012 and 2016) were not considered in these analyses, but individuals who had experienced these events before the study period were included. Transitions are shown in a Sankey diagram developed using the online tool SankeyMATIC (https://sankeymatic.com/).
Compliance with Ethics Guidelines
The linkage of individual-level data from multiple national registers was approved by the Ethical Review Board, Lund (dnr 2018-349) and the Swedish Ethical Review Authority (dnr 2019-01260). Informed consent is not required for secondary use of register data.
Results
Baseline Characteristics
Baseline characteristics for each subgroup and their matched controls are shown in Table 2. After matching, 80,305 individuals with ASCVD and 80,305 controls were included. In total, 15,397 individuals with PAD, 17,539 individuals with previous stroke and 25,729 individuals with previous MI were included, with the same number of matched controls for each subgroup. Mean baseline age was 67–68 years. In the MI subgroup and matched controls, 26% of individuals were women; in the other subgroups and control groups, 32–34% of individuals were women. Individuals with ASCVD, PAD, previous stroke or previous MI were more likely to have baseline comorbidities, including heart failure and renal disease, than matched controls. Individuals with MI had the largest disparity between cases and controls in terms of baseline heart failure (previous MI, 14.6%; controls, 2.3%), whereas the subgroup with PAD had the highest proportion of individuals with baseline renal disease (PAD, 38% had kidney disease at baseline and 5% had end-stage renal disease; controls, 12% had kidney disease at baseline and 0.8% had end-stage renal disease).
Total Costs
In all groups, the presence of a cardiovascular condition in addition to T2D was associated with increased costs. The largest disparity between cases and controls was observed in the PAD analyses: total mean annual costs per person were €14,785 (SEM 89) for PAD and €5526 (SEM 48) for controls. Total costs were €11,397 (SEM 68) for individuals with previous stroke and €5294 (SEM 43) for controls; €10,730 (SEM 31) for individuals with ASCVD and €5797 (SEM 22) for controls; and €10,342 (SEM 55) for individuals with previous MI and €5930 (SEM 39) for controls.
Direct Costs
In individuals with PAD, direct costs accounted for the largest proportion of mean annual total costs (54%), whereas in all other subgroups and control groups, direct costs contributed nearly half (40–47%) of total costs. Direct costs for individuals with ASCVD or in each subgroup were higher than for their matched controls (Fig. 1). Notably, mean annual per-person direct costs for individuals with T2D and PAD were more than threefold higher than for matched controls with T2D and no PAD.
Mean direct (a) and indirect (b) costs per person per year during the study period in individuals with T2D and ASCVD, PAD, previous stroke or previous MI and matched controls without ASCVD. Direct costs were calculated among all individuals. Indirect costs were assumed to be zero for individuals aged 66 years or older. In panel a, constituent costs and percentages do not sum exactly to totals due to rounding. ASCVD atherosclerotic cardiovascular disease, MI myocardial infarction, PAD peripheral artery disease, T2D type 2 diabetes
The composition of direct costs was relatively similar across all groups. Inpatient costs contributed 71–75% of direct costs in cases and 68–70% in controls; outpatient costs contributed 14–20% in cases and 14–15% in controls; and drug costs contributed 9–13% in cases and 17–18% in controls. Outpatient costs made a larger contribution to direct costs in individuals with PAD than in other groups (20% of direct costs) and inpatient costs made a larger contribution in individuals with previous stroke (75%).
Mean cumulative direct costs over the entire study period amounted to €40,013 for individuals with PAD, €26,736 for individuals with previous stroke, €26,153 for individuals with ASCVD and €25,446 for individuals with previous MI (Fig. 2). The equivalent costs in each control group were approximately €14,000.
Mean cumulative direct, indirect and total costs per person during the study period in individuals with T2D and a ASCVD, b PAD, c previous stroke or d previous MI and matched controls without ASCVD. Data are mean cumulative costs in each year during the study period (2012–2016) among individuals alive in each year. Indirect costs for individuals aged 66 years or older were not accounted for. ASCVD atherosclerotic cardiovascular disease, MI myocardial infarction, PAD peripheral artery disease, T2D type 2 diabetes
Indirect Costs and Work Absence
The numbers of individuals in each group aged less than 66 years, for whom indirect costs were estimated, are shown in Table S4 in the Supplementary Material. Mean annual per-person indirect costs were higher for individuals with ASCVD or in each subgroup than for matched controls. The greatest disparities were observed for PAD and previous stroke, for which costs for cases were double those for controls, whereas there were more moderate differences between cases and controls for ASCVD and previous MI.
Mean cumulative indirect costs over the study period were highest for individuals with PAD (€29,064 vs. €12,143 for controls) and individuals with previous stroke (€28,457 vs. €11,009 for controls; Fig. 2).
Across the entire study period, individuals with ASCVD, PAD, previous stroke or previous MI spent more days absent from work than matched controls (Table S4 in the Supplementary Material). Overall, 22% of working age individuals with ASCVD had 360 or more days absent from work per year, compared with 12% of controls. The equivalent proportions were 26% and 11% for PAD; 33% and 12% for previous stroke; and 18% and 12% for previous MI.
Direct Costs Following the First Observed Occurrence of ASCVD
Available cost data throughout the study period were compared between cases with a first occurrence of ASCVD in 2011 and matched controls (Fig. S1 and Table S5 in the Supplementary Material). Before 2011, direct costs were slightly higher for cases than controls, except in the MI analyses in which costs were similar. Direct costs increased sharply upon the occurrence of ASCVD (n = 6387 with the first occurrence in 2011), PAD (n = 1090), stroke (n = 1300) or MI (n = 1587; Table S5 in the Supplementary Material), and remained well above baseline for the remainder of the study period, whereas costs in each control group increased only gradually over the study period. For ASCVD overall, costs increased from €2051 in 2010 to €14,158 in 2011, and were €5454 in 2012. There were particularly large cost increases immediately following the occurrence of stroke (2010, €1972; 2011, €18,132; 2012, €4564) and MI (2010, €1218; 2011, €17,506; 2012, €4677). In contrast, PAD had a lower cost impact on first occurrence, but was associated with higher costs in the years preceding the first occurrence, and greater cost elevations for a longer period of time (2010, €2974; 2011, €11,471; 2012, €8225; 2016, €6798).
Mortality and Cardiovascular Events
All conditions were associated with increased risks for mortality. Overall, 23% of individuals with ASCVD, but only 12% of controls, died during the study period. These proportions were the same for previous MI versus controls. For PAD and previous stroke, the disparities between cases and controls were greater (36% vs. 13% and 30% vs. 13%, respectively; Table S6 in the Supplementary Material).
All conditions were associated with increased risks for stroke or MI during the study period (Fig. 3). Individuals who had experienced previous stroke had a threefold higher chance of experiencing stroke during the study period than controls, and individuals with PAD or previous MI had more than threefold higher chances of experiencing MI during the study period than controls. The increased risks for occurrence of these cardiovascular conditions are likely to explain increases in both direct and indirect costs. Each year, 1–2% of individuals had a new MI and 0.8–1.4% had a new stroke, transitioning from a state with no events during the study period (Fig. 4). Mortality increased from 4.5% to 4.8%, with nearly 23% of individuals deceased by the end of the study period (Fig. 4).
Hazard ratios for all-cause mortality, cardiovascular events and full-time early retirement in individuals with T2D and ASCVD, PAD, previous stroke or previous MI and matched controls without ASCVD. Hazard ratios for early retirement were calculated in the subset of the study sample who were aged less than 66 years and not already in full-time retirement. ASCVD atherosclerotic cardiovascular disease, CI confidence interval, HR hazard ratio, MI myocardial infarction, PAD peripheral artery disease, T2D type 2 diabetes
Transitions during 2012–2016 to four alternative states or remaining in a state with no new events, for individuals with T2D and ASCVD. The sample size decreased each year owing to emigration and loss to follow-up. ASCVD atherosclerotic cardiovascular disease, MI myocardial infarction, T2D type 2 diabetes
Early Retirement
For ASCVD, PAD, previous stroke or previous MI, cases were more likely than controls to be in full-time early retirement at baseline (Table 2), and all conditions were associated with a higher likelihood of entering full-time early retirement during the study period, compared with controls (Fig. 3). Notably, individuals with previous stroke were more than three times more likely to enter full-time early retirement between 2012 and 2016 than controls.
Discussion
In these comprehensive analyses, we assessed the effects of ASCVD, PAD, stroke and MI in a large population of individuals with T2D. By including a wide range of outcomes, we demonstrated the impact of these conditions on individuals, healthcare systems and society.
Total costs were nearly double for individuals with T2D and ASCVD than for controls, and the impacts of PAD and stroke on total costs were each greater than the impact of ASCVD more broadly. The disparity between the cost impact of PAD and the impacts of ASCVD, stroke or MI was largely driven by higher direct healthcare costs for PAD. Although inpatient costs were the largest contributor to direct costs in all subgroups and controls, outpatient costs contributed a larger proportion of direct costs in individuals with PAD (20%) than in the other groups. PAD had a lower direct cost impact in the year that it occurred, but resulted in a more prolonged elevation of direct costs, relative to the other conditions. Individuals with PAD also had relatively higher mortality.
Indirect costs were broadly similar across the group with ASCVD and the subgroups, and were higher for cases than for controls. In all groups, apart from individuals with PAD, indirect costs accounted for more than half of total costs. Of the conditions assessed, stroke had the largest impact on work absence. It should be noted that mean indirect costs were calculated for the full populations in our analyses, but in people aged 66 years or older these costs were not accounted for. Therefore, the impact of ASCVD on indirect costs in individuals of working age is even higher than the estimates presented here.
The impact of ASCVD on both direct and indirect costs in T2D in these analyses is partly attributable to the associated higher risks for stroke and MI, compared with T2D alone. As well as requiring both acute and long-term treatment, resulting in direct healthcare costs, stroke and MI impact affected individuals’ ability to return to work. A proportion of the higher costs in T2D with ASCVD observed in this study can therefore be attributed to a higher prevalence of concomitant conditions, such as renal disease, compared with controls. The study design limited the matching variables to year of birth, sex and educational level; consequently, the observed differences in baseline comorbidities were expected.
Previous studies have also demonstrated the impact of cardiovascular conditions on direct healthcare costs in populations with T2D. A systematic review published in 2018 found that at a country level, CVD, heart failure or MI contributed 20–49% of the total direct costs of treating T2D and at the individual level, median annual per-person costs were 59–322% higher for individuals with T2D and CVD, coronary artery disease, heart failure or stroke, compared with those for individuals with T2D without CVD [19]. A more recent systematic review of data from Spain reported that direct costs in hospitalised individuals with T2D were more than 50% higher in individuals with CVD (including coronary heart disease, stroke, PAD and heart failure) than in those without CVD [28]. Very few studies, however, have examined the impact of ASCVD on indirect costs, owing in part to limitations in data availability. This highlights the need for comprehensive studies such as ours, in which we showed that lost productivity and direct healthcare costs are equally important contributors to the overall cost impact of ASCVD in T2D.
The prevalence of T2D is increasing in many countries [29], as improved diabetes management and new treatments have reduced the detrimental effects of the condition and improved survival [30]. Globally, ageing populations and changing living conditions contribute to the growing prevalence of T2D [31] and increasing numbers of people living with CVD [32]. The results of the present study support continued and increased early prevention of ASCVD in the management of T2D. In addition to the guideline-recommended prescription of GLP-1 RAs or SGLT2is for individuals with T2D and CVD or high CVD risk [10,11,12], treatments that can lower LDL cholesterol (LDL-C) also reduce the risk of ASCVD in individuals with or without T2D. Statins are the first line of LDL-C-lowering therapy [33], and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are a therapeutic option for individuals who do not respond to statins [34]. Quantification of the precise benefits that could be gained by optimal cardiovascular risk management in people with T2D was outside the scope of our study, but a post hoc analysis of data from the CAPTURE study has attempted to address this question by estimating the life years that could be gained via the use of appropriate medications [35]. The societal impact of ASCVD demonstrated in our analyses suggests that public health initiatives aimed at limiting modifiable risk factors associated with CVD and T2D should also be a key health policy priority.
Access to a large national sample allowed us to perform propensity score matching and identify controls with the same age, sex and level of education but without ASCVD. Therefore, we have been able to obtain accurate estimates of multiple impacts of ASCVD in T2D, including in subgroups with specific conditions. However, additional research is needed to assess the disease courses of these conditions. The study design and data source meant that people in early disease stages without an ASCVD event in a hospital setting were not included, and the elderly population were underrepresented in the study sample. Some relevant factors were not considered in the analyses, such as T2D medication, clinical endpoints such as glycated haemoglobin levels or blood pressure, and socioeconomic factors such as occupation. Not all of these data were available in a structured form in our database; however, future studies using alternative data sources to examine these contributors to the disease course of T2D and ASCVD would be valuable. Furthermore, although the findings of our study hint at the wider humanistic burden of ASCVD in T2D, a full exploration of the impacts on the quality of life of affected individuals and caregivers is warranted.
Conclusions
This study demonstrates that ASCVD is associated with considerable additional costs, morbidity and mortality in individuals with T2D, compared with T2D alone. Work absence and hospitalisation were the major contributors to economic costs, and PAD was the most costly manifestation of ASCVD examined in the analyses. Considerable economic impacts could be avoided by timely management of cardiovascular risk in T2D, and future initiatives focusing on both treatment and prevention should be implemented.
References
Bernfort L, Husberg M, Wirehn AB, et al. Disease burden and healthcare costs for T2D patients with and without established cardiovascular disease in Sweden: a retrospective cohort study. Diabetes Ther. 2020;11:1537–49.
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1046–81.
Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–6.
Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA. 2004;292:2495–9.
Echouffo-Tcheugui JB, Xu H, Matsouaka RA, et al. Diabetes and long-term outcomes of ischaemic stroke: findings from Get With The Guidelines-Stroke. Eur Heart J. 2018;39:2376–86.
Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—mechanisms, management, and clinical considerations. Circulation. 2016;133:2459–502.
Raghavan S, Vassy JL, Ho YL, et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 2019;8:e011295.
American Diabetes Association. 9. Cardiovascular disease and risk management: standards of medical care in diabetes—2018. Diabetes Care. 2017;41:S86–104.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323.
Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42:3227–337.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;2022(45):2753–86.
Mosenzon O, Alguwaihes A, Leon JLA, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20:154.
SWEDEHEART. Annual report 2021 (English). Issued in 2021. https://www.ucr.uu.se/swedeheart/dokument-sh/arsrapporter-sh. Accessed 11 Jan 2023.
Weng W, Tian Y, Kong SX, et al. Impact of atherosclerotic cardiovascular disease on healthcare resource utilization and costs in patients with type 2 diabetes mellitus in a real-world setting. Clin Diabetes Endocrinol. 2020;6:5.
Bauersachs R, Zeymer U, Brière JB, Marre C, Bowrin K, Huelsebeck M. Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther. 2019;2019:8295054.
Marrett E, daCosta DiBonaventura M, Zhang Q. Burden of peripheral arterial disease in Europe and the United States: a patient survey. Health Qual Life Outcomes. 2013;11:175.
Thiruvoipati T, Kielhorn CE, Armstrong EJ. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6:961–9.
Einarson TR, Acs A, Ludwig C, Panton UH. Economic burden of cardiovascular disease in type 2 diabetes: a systematic review. Value Health. 2018;21:881–90.
Kamil S, Sehested TSG, Carlson N, et al. Diabetes and risk of peripheral artery disease in patients undergoing first-time coronary angiography between 2000 and 2012—a nationwide study. BMC Cardiovasc Disord. 2019;19:234.
Olesen KKW, Gyldenkerne C, Thim T, Thomsen RW, Maeng M. Peripheral artery disease, lower limb revascularization, and amputation in diabetes patients with and without coronary artery disease: a cohort study from the Western Denmark Heart Registry. BMJ Open Diabetes Res Care. 2021;9:25.
Kjellberg J, Tikkanen CK, Bagger M, Gæde P. Short-term societal economic burden of first-incident type 2 diabetes-related complications - a nationwide cohort study. Expert Rev Pharmacoecon Outcomes Res. 2020;20:577–86.
Kristófi R, Bodegard J, Norhammar A, et al. Cardiovascular and renal disease burden in type 1 compared with type 2 diabetes: a two-country nationwide observational study. Diabetes Care. 2021;44:1211–8.
Persson S, Johansen P, Andersson E, et al. Days absent from work as a result of complications associated with type 2 diabetes: evidence from 20 years of linked national registry data in Sweden. Diabetes Obes Metab. 2020;22:1586–97.
Andersson E, Persson S, Hallén N, et al. Costs of diabetes complications: hospital-based care and absence from work for 392,200 people with type 2 diabetes and matched control participants in Sweden. Diabetologia. 2020;63:2582–94.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657.
Artime E, Romera I, Díaz-Cerezo S, Delgado E. Epidemiology and economic burden of cardiovascular disease in patients with type 2 diabetes mellitus in Spain: a systematic review. Diabetes Ther. 2021;12:1631–59.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98.
Jansson SP, Fall K, Brus O, et al. Prevalence and incidence of diabetes mellitus: a nationwide population-based pharmaco-epidemiological study in Sweden. Diabet Med. 2015;32:1319–28.
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes—Global Burden of Disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107–11.
Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Joseph JJ, Deedwania P, Acharya T, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation. 2022;145:e722–59.
Handelsman Y, Lepor NE. PCSK9 inhibitors in lipid management of patients with diabetes mellitus and high cardiovascular risk: a review. J Am Heart Assoc. 2018;7:25.
Ostergaard HB, Humphreys V, Hengeveld EM, et al. Cardiovascular risk and lifetime benefit from preventive treatment in type 2 diabetes: a post hoc analysis of the CAPTURE study. Diabetes Obes Metab. 2023;25:435–43.
Acknowledgements
Funding
This study was supported by a Grant from Novo Nordisk A/S, Søborg, Denmark to the Swedish Institute for Health Economics. The Rapid Service Fee was funded by Novo Nordisk A/S.
Medical Writing, Editorial, and Other Assistance
The authors would like to acknowledge Barbara Xella PhD, Helen Schofield BSc and Caroline Freeman PhD of Oxford PharmaGenesis, Oxford, UK for medical writing support, which was funded by Novo Nordisk A/S.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to study design, data interpretation, and critical review and revision of the manuscript. KSC and KN analysed the data. All authors read and approved the final manuscript for publication.
Prior Presentation
The results of this study were presented in part at the 58th European Association for the Study of Diabetes Annual Meeting, 19–23 September 2022, Stockholm, Sweden.
Disclosures
Katarina Steen Carlsson and Kristoffer Nilsson are employees of the Swedish Institute for Health Economics. Katarina Steen Carlsson is also an employee of the Department of Clinical Sciences, Malmö, Lund University, Lund, Sweden. Neither has received payment outside of their normal salaries at the Swedish Institute for Health Economics related to the subject matter of this work. Mads Faurby and Michael Lyng Wolden are employees of Novo Nordisk A/S.
Compliance with Ethics Guidelines
This study was performed in accordance with the Declaration of Helsinki (1964), and with permission from the database owners. The linkage of individual-level data from multiple national registers was approved by the Ethical Review Board, Lund (dnr 2018-349) and the Swedish Ethical Review Authority (dnr 2019-01260). Informed consent is not required for secondary use of register data.
Data Availability
Subject-level data from national registers hosted by Swedish national authorities are available for research after formal evaluation of the research protocol by the Swedish Ethical Review Authority and by the respective national authorities providing data. Permission to conduct research is granted on a case-by-case basis to a limited number of named people. The secondary individual-level data of the study cannot be shared by the authors for this reason.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Steen Carlsson, K., Faurby, M., Nilsson, K. et al. Atherosclerotic Cardiovascular Disease in Type 2 Diabetes: A Retrospective, Observational Study of Economic and Clinical Burden in Sweden. Diabetes Ther 14, 1357–1372 (2023). https://doi.org/10.1007/s13300-023-01430-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01430-4