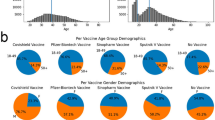
Abstract
Introduction
Since early 2020 the whole world has been challenged by the SARS-CoV-2 virus (COVID-19), its successive variants and the associated pandemic caused. We have previously shown that for people living with type 2 diabetes (T2DM), the risk of being admitted to hospital or dying following a COVID-19 infection progressively decreased through the first months of 2021. In this subsequent analysis we have examined how the UK COVID-19 vaccination programme impacted differentially on COVID-19 outcomes in people with T1DM or T2DM compared to appropriate controls.
Methods
T1DM and T2DM affected individuals were compared with their matched controls on 3:1 ratio basis. A 28-day hospital admission or mortality was used as the binary outcome variable with diabetes status and vaccination for COVID-19 as the main exposure variables.
Results
A higher proportion of T1DM individuals vs their controls was found to be vaccinated at the point of their first recorded positive COVID-19 test when compared to T2DM individuals vs their controls. Regarding the 28-day hospital admission rate, there was a greater and increasing protective effect of subsequent vaccination dosage (one, two or three) in mitigating the effects of COVID-19 infection versus no vaccination in T1DM than in T2DM individuals when compared with matched controls. Similar effects were observed in T2DM for death. Across both diabetes and non-diabetes individuals, those at greater socio-economic disadvantage were more likely to test positive for COVID-19 in the early phase of the pandemic. For T2DM individuals socio-economic disadvantage was associated with a greater likelihood of hospital admission and death, independent of vaccination status. Age and male sex were also independently associated with 28-day hospital admission in T2DM and to 28-day mortality, independent of vaccination status. African ethnicity was also an additional factor for hospital admission in people with T2DM.
Conclusion
A beneficial effect of COVID-19 vaccination was seen in mitigating the harmful effects of COVID-19 infection; this was manifest in reduced hospital admission rate in T1DM individuals with a lesser effect in T2DM when compared with matched controls, regarding both hospital admission and mortality. Socio-economic disadvantage influenced likelihood of COVID-19 confirmed infection and the likelihood of hospital admission/death independent of the number of vaccinations given in T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
People with diabetes are at particularly high risk of becoming seriously unwell and being hospitalised after contracting SARS-CoV-2 (COVID-19) |
This study set out look at how the UK vaccination programme impacted differentially on COVID-19 outcomes in people with T1DM or T2DM compared with appropriate controls |
For 28-day hospital admission rates in T1DM following the first positive COVID-19 test, there was a greater effect of increasing vaccination dosage (one, two or three) in mitigating the effects of COVID-19 infection vs no vaccination than seen in T2DM individuals when compared to controls |
Socio-economic deprivation in T2DM individuals was associated with greater likelihood of hospital admission and death, independent of vaccination status |
In T2DM, age and male sex were both independently associated with increased 28-day hospital admission and 28-day mortality, independent of vaccination status. African ethnicity was an additional factor for 28-day hospital admission in T2DM |
The COVID-19 vaccination programme afforded protection for people with T1DM and T2DM with a clear greater benefit in T1DM than in non-T1DM individuals of a similar age and sex |
Introduction
Since early 2020 the whole world has been challenged by the SARS-CoV-2 virus (COVID-19) and its associated pandemic [1]. This situation has been further complicated with the successive rise of subsequent viral variants with varying clinical and transmission properties [1]. People with diabetes are known to be at a higher risk of becoming unwell and dying following COVID–19 infection when compared to people living without diabetes [2,3,4]. Higher odds ratios (OR) have been reported for in-hospital COVID-19-related death for people with type 2 diabetes (T2DM) in the UK [4]. A subsequent body of work is now emerging in relation to why people with diabetes are more likely to become seriously unwell following a COVID-19 infection and why they are admitted into hospital with COVID-19 vaccination, sometimes itself a factor in hospital admission.
Utilising the Greater Manchester Care Record (GMCR) we have recently shown that for people with T2DM, following confirmed infection with COVID-19, a number of factors are associated with 28-day post-confirmed increased COVID-19-positive test hospital admission in T1DM/T2DM and increased mortality in individuals with T2DM [5, 6]. This analysis reported that prescription of metformin, SGLT2is or GLP-1 agonists and non-smoking status appeared to be associated with a reduced risk of hospital admission/death for people with T2DM. Age, male sex and social disadvantage were associated with an increased risk of death/hospital admission in people with T2DM [5, 6]. Hippisley-Cox et al. [7] independently described a risk model for hospital admission following COVID-19 infection, using pooled National Health Service (NHS) primary care data with age being highlighted as a predominant risk factor for death following COVID-19 infection [7, 8].
From December 2020, the number of T2DM individuals vaccinated against COVID-19 has steadily risen across the UK for initially the first vaccination and then subsequent vaccination [9]. There are now more than three vaccines available in the UK with a high proportion of the population now triple vaccinated or even quadruple vaccinated [10].
It is important to appreciate how much effect vaccination against COVID-19 has had to reduce the risk health outcomes for people living with diabetes. Another important question is whether being vaccinated against COVID-19 offers the same protection or different protection for individuals with diabetes compared to individuals free of diabetes. Furthermore, there have been significant differences in vaccine uptake across the UK [11] and it is therefore important to investigate what demographic factors may be influencing this.
The aims of this study were therefore to determine the health trajectory of vaccinated vs non-vaccinated individuals with T1DM or T2DM. For this we considered that critical outcomes following a COVID-19-positive test would be hospital admission rate and death. Our primary aim was to ascertain the effect of COVID-19 vaccination on reducing the adverse consequences of COVID-19 infection following on from our previous papers [5, 6]. A further aim was to determine vaccination rates within socially disadvantaged communities in Greater Manchester using the Townsend Index [12] as the measure of social disadvantage.
Methods
Vaccination status was determined from general practice records. We applied 28-day hospital admission or mortality as the binary outcome variable and diabetes status as the main exposure variable in relation to vaccination for COVID-19. Study participants included all people who had a recorded diagnosis of T1DM or T2DM living in the UK Greater Manchester conurbation (total population 2.85 million) who were alive on 1 January 2020 and who had a proven COVID-19 infection, based on a recorded positive test noted in the general practice record. Each individual with diabetes (T1DM or T2DM) was matched with three controls—that is three people without diabetes matched for age and gender who had also tested positive within a 28-day period for a COVID-19 infection.
Data were de-identified at source and were extracted from the Greater Manchester Care Record (GMCR) database [13]. The follow-up period started on 1 January 2020 and ended on 28 February 2022. The GMCR pools information from all general practices across the conurbation. The project was approved and overseen by Health Innovation Manchester [14].
The data were put through a rigorous checking and data cleaning process where all values were ‘sense checked’ for credible physiological ranges and internal clinical and demographic logic (e.g. dates of birth, height, weight, BMI, biomarker ranges). BMI was included only if recorded within 6 months of the positive COVID-19 test. Vaccination dates for the first and second vaccines administered were recorded. Statistical analyses were performed on the final data set to investigate the potential risk factors contributing to increased likelihood for hospital admission in diabetes following infection with COVID-19. Social disadvantage was described by Townsend score [12]. A higher Townsend index equates to greater social disadvantage. The 2011 census was used to define ethnicity.
To take account of COVID-19-positive status being confirmed after hospital admission, we included in our analysis all individuals with a COVID-19 virus-positive test within 48 h of admission.
Following cleaning, the data extracted were split into two cohorts.
-
Those with T1DM and their controls (1:3 matching).
-
Those with T2DM and their controls (1:3 matching).
Statistics
Digital health records often contain missing data, particularly for medications and diagnoses. We assumed that missing data for these variables meant individuals were not on that medication or had a specific diagnosis. Imputation in relation to the comparison between diabetes and non-diabetes individuals was not possible because of the degree of difference in availability of anthropometric and metabolic variables between the two groups. In other words many people without diabetes had very limited data available. Therefore a complete case analysis was conducted. Comparison between continuous variables was performed by ANOVA.
All substantive modelling was by logistic regression, with 28-day hospital admission or mortality as the binary outcome variable and diabetes status as the main exposure variable. Other variables were adjusted for in specific models, as detailed below. Townsend score comparisons were relative to a Townsend score of 1.
To investigate potential factors associated with admission in diabetes patients, we analysed the T1DM and T2DM individuals, without the matched individuals, separately. To do so, we used univariate logistic regression, considering each possible factor in turn for the two groups separately.
We then studied whether the difference in risk of admission/death between patients with diabetes and patients without diabetes (and between those vaccinated and those not-vaccinated) was explained by other measured factors. To do so we analysed each diabetes group with their matched cohort and compared the odds ratio (OR) of diabetes in a model including other relevant variables.
Finally, we fitted a fully adjusted multi-variable model separately for T1DM and T2DM but including each respectively matched cohort to measure the extent of attenuation of the diabetes OR when all additional factors are accounted for.
All analyses were undertaken in R (version 3.6.2) (R Foundation for Statistical Computing, Vienna, Austria). Data presented are mean ± standard deviation, unless stated otherwise.
Results
In the population examined, 1659 T1DM individuals with a mean/standard deviation (SD) age of 38.0 (SD 17.6) years had a confirmed positive COVID–19 test. Furthermore, 20,547 individuals with T2DM with a mean age of 62.5 (SD 14.2) years were confirmed to have a positive COVID-19 test (Tables 1 and 2).
In the early phase of the COVID-19 pandemic at least from April 2020 until April 2021, for both T1DM and T2DM individuals, greater socio-economic disadvantage was associated with a greater likelihood of test-positive status for COVID-19.
There was no significant difference in the proportions of people with T1DM or matched controls in relation to socio-economic status as measured by Townsend index [12]. However, there were higher proportions of people with T2DM compared to matched controls in the next to lowest quartile (22.6% vs 20.6%) and most disadvantaged Townsend index quartile (35.6% vs 22.4%).
Mean body mass index (BMI) was similar in T1DM individuals and their matched controls. Mean BMI was higher in T2DM individuals (32.0, SD 7.0 kg/m2) when compared with matched controls (28.5 [SD] 6.1 kg/m2).
A higher proportion of the T1DM individuals (19.2%) than controls (16.3%) had received two vaccinations at the point of their first COVID-19-positive test. A similar finding was observed for three vaccinations at the point of the first COVID-19-positive test (13.7% vs 10.5%).
Regarding distribution of ethnicity, substantially more individuals of (South) Asian heritage were present in the T2DM group (19.4%) when compared to controls (6.1%). A higher proportion of individuals with Caucasian heritage identified in the T1DM DM group when compared to controls. There was no difference in the proportion of T1DM individuals documented COVID-19-positive vs -non-T1DM individuals with a history of severe enduring mental illness, but there was a higher proportion of T2DM COVID-19 test-positive individuals with SMI vs matched test-positive controls.
Twenty-eight-day Hospital Admission Rates
The 28-day hospital admission rate was higher in T1DM individuals (6.4%) compared to matched controls (4.8%) (p < 0.001) (Table 1). For T2DM individuals the hospital admission rate was 14.5% vs 8% in matched controls (p < 0.001) (Table 2).
Twenty-eight-day Mortality Rates
Twenty-four deaths were identified in the T1DM group within 28 days of a confirmed COVID-19 test (1.4%) vs 0.7% in matched controls (p < 0.007). There were 1316 deaths within the T2DM individuals within 28 days of a confirmed COVID-19 test giving a significant mortality rate difference of 6.4% vs 4.8% in matched controls (p < 0.001).
Logistic Regression of Factors Associated with Admission in T1DM Patients and T2DM Patients
While a decrease in likelihood of admission was reduced by increasing number of vaccinations vs no vaccination (prior to the first COVID-19-positive test in both T1MD and T1DM controls), the relative impact of vaccination on hospital admission was greater in T1DM individuals (Table 3). No difference was seen between T2DM and T2DM controls in terms of impact of increasing number of vaccinations on likelihood of hospital admission (Table 4). However, baseline admission rate (see Table 2) was much higher in T2DM individuals with therefore greater net impact in terms of absolute number of reduced admissions following COVID-19 vaccination.
As seen in Table 4, several factors were likely to increase the likelihood of admission independent of vaccination status in T2DM people, notably a more deprived Townsend score, a higher BMI, current smoking status and African heritage. In contrast only age was observed as a risk factor for hospital admission in T1DM individuals in addition to vaccination status. A higher BMI was associated with increased hospital admission rate in T1DM controls but not people with T1DM. For T2DM control individuals South Asian heritage was an independent risk factor for 28-day hospital admission (Table 4), as was elevated BMI and current smoking status but not African heritage.
Logistic Regression of Factors Associated with 28-Day Mortality in T1DM and T2DM Patients
With only 24 deaths observed in the T1DM group it was not possible to determine any influence of any specific risk factors on outcome following a positive COVID-19 test. In T2DM individuals there was a gradation of reduced mortality with increasing numbers of COVID-19 vaccinations 1–3. A similar effect was seen in aged matched non-T2DM individuals. For T2DM individuals, in logistic regression analysis, age, male sex and socio-economic disadvantage were associated with in increased likelihood of death (Table 5) but not elevated BMI or current smoking status. In T2DM controls it was actually a lower BMI that was associated with an increased mortality rate with again no independent effect of smoking.
Second COVID-19 Infection and Vaccination Status
For those confirmed COVID-19 positive on a second occasion, for T1DM individuals, 86.2% were unvaccinated as were 90.7% of matched non-T1DM controls and, for T2DM individuals, 86.3% were unvaccinated compared with 86.5% of matched non-T2DM controls.
Discussion
In this study we have identified a significant beneficial effect of COVID-19 vaccination for mitigating the serious more immediate effects of COVID-19 infection, manifest in hospital admission rate, in T1DM individuals. This was seen to a lesser extent in T2DM when compared with control non-T2DM individuals. Socio-economic disadvantage increased the likelihood of hospital admission/death independent of number of vaccinations in T2DM which again highlights the influence of socio-economic factors on outcomes following a COVID-19 infection in this group of people. A higher BMI and current smoking status increased the likelihood of hospital admission independent of other factors in both T2DM and T2DM control individuals but did not increase mortality rate. Interestingly in T2DM controls, a lower BMI was associated with a higher mortality rate, which may reflect the fact that a lower BMI can be a marker of overall ill health. Furthermore, in T1DM controls, a higher BMI was associated with an increased hospital admission rate but not in T1DM individuals.
While we have shown a gradient of increased benefit of multiple vaccination (2 and 3 vaccinations) on 28-day hospital admission rate in T1DM, a factor that we cannot account for in this study is the decreasing severity of COVID-19 infections in many but not all people over time [15]. The greatest decrease in both hospital admission and death after COVID-19 infection was in relation to the third vaccination but this may associate with the decreasing ‘dangerousness’ of the COVID-19 virus over time.
Nevertheless, the fact that we see less of a gradient of increased protection in T2DM vs matched controls with an increased number of vaccinations over time does suggest that what is seen in T1DM may represent a true protective effect regarding hospital admission of vaccination in this group. The significant reduction in admission rate in T2DM vs non-T2DM individuals, while similar, is on the background of a much higher admission rate in T2DM (Table 2).
T1DM individuals were more likely to have had their COVID-19 vaccinations at the time of their first COVID-19-positive test with much less of difference for T2DM individuals vs their matched contemporaries in relation to timing of vaccination. This is likely an age-related phenomenon because of age stratification in the COVID-19 vaccination programme England and the fact that younger individuals without a co-existing chronic condition were the last adult age group to be offered COVID-19 vaccination [13]. However, given the much higher admission rate in T2DM individuals following a COVID-19 infection the relative benefit of COVID-19 vaccination remains.
Factors increasing the likelihood of hospital admission were age in T1DM individuals and, in T2DM individuals, male sex, age, more deprived Townsend score and African heritage (compared with non-T2DM controls where Asian heritage was an independent risk factor for admission). The difference between T2DM and non-T2DM individuals in predisposing ethnic factors may relate to the larger sample size on the basis of 3:1 matching of controls to people with T2DM, given that South Asian heritage has been reported to be an independent risk factor for serious illness after COVID-19 infection in other studies [16, 17]. In T1DM only age was related to likelihood of 28-day hospital admission in addition to vaccination status—this may be associated with the relatively low numbers of people with T1DM who tested positive. However, the benefits of an increasing number of COVID-19 vaccinations is clear for the T1DM group. Previously, Barron et al. reported the significant risks of COVID-19 infection in terms of adverse health consequences in T1DM as well as T2DM individuals [4].
It should be stated that multiple unresolved issues including preferred vaccine type/combination of vaccines, vaccine efficacy and durability and frequency of administration remain unresolved and need to be addressed through future research. Our findings, while related to a population in one part of the UK, do build on previous reports from the same background population [5, 6].
It should also be pointed out that we do not have the cases and control in the same analysis; we ran separate analyses. At no point were the cases and controls combined in the same analysis. Propensity matching was not applicable here because the data were extracted in a matched form and further propensity matching would result in a much smaller subset of patients than the one that we were able to use. Furthermore, we have reported that BMI is a major contributor to the excess risk seen in people with T2DM as it also is in non-diabetes individuals. We do not therefore feel that there is the need to match individuals for BMI as well as age and gender in this paper.
A significant step in primary prevention of infections is timely and appropriate vaccination [18]. Routine vaccination against influenza is recommended in patients with DM [19]. Although prior studies have shown impaired antibody response to influenza and hepatitis B vaccines in patients with DM (with recent advances in the development of vaccines, people with DM are able to mount an appropriate immune response post-vaccination) [20]. Adults with T2DM considerably benefit from influenza vaccination in terms of reduction in any complications, hospitalizations and death [21]. Hence, these vaccinations have been included in the standard care for DM patients. We believe that the data presented here support the proposal that COVID-19 vaccination should now be offered to people with diabetes as a designated at risk group, irrespective of age.
Strengths/Limitations
A limitation common to all COVID-19 research is that during the first few months of the pandemic there was limited capacity to test for COVID-19 testing, and so the true prevalence is unknown and certainly for the early months of the COVID-19 pandemic can only be estimated [22]. Thus, there is the likelihood of there being an underestimate of the total number of COVID-19-positive test results, hence the high 28-day hospital admission rate and mortality rate compared to Office of National Statistics (ONS) results [23]. However, there is no reason to suspect that this would affect diabetes vs non-diabetes individuals differentially. A strength of this study is that by matching our cohort on the date of positive coronavirus test, as well as age and sex, we are able to correct for this and focus on the differences between the diabetes T1DM and T2DM cohorts and the general population.
A further limitation is that the data only cover the area of Greater Manchester in the UK and we have relied on general practice record coded diagnoses and COVID-19 recorded test results rather than other sources of that data.
Regarding additional relevant variables such as HbA1c, comorbidities (e.g. hypertension, coronary heart disease, and stroke) and complications (e.g. diabetes nephropathy, diabetes retinopathy, diabetes foot), we did not add them here as this article follows on from analyses as already reported in this journal that did not include vaccination as a variable. It is planned that these variables will be included in our next paper.
Conclusion
We have found a significant beneficial effect of COVID-19 vaccination in mitigating the effects of COVID-19 infection, manifest in hospital admission rate, in T1DM individuals with a possibly lesser effect in T2DM when compared with non-diabetes individuals regarding both hospital admission and mortality. Socio-economic disadvantage influenced the likelihood of hospital admission/death independent of number of vaccinations in T2DM. Going forward we suggest that further boostering of COVID-19 vaccination programmes might include T1DM/T2DM individuals as a priority group and continue to facilitate access to vaccination for more disadvantaged socio-economic groups.
References
https://www.who.int/health-topics/coronavirus: Accessed 24 July 2022
Tadic M, Cuspidi C, Sala C. COVID-19 and diabetes: Is there enough evidence? J Clin Hypertens (Greenwich). 2020;22:943–8.
Katulanda P, Dissanayake HA, Ranathunga I, Ratnasamy V, Wijewickrama PSA, Yogendranathan N, Gamage KKK, de Silva NL, Sumanatilleke M, Somasundaram NP, Matthews DR. Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature. Diabetologia. 2020;63:1440–52.
Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar N, Wareham NJ, Young B, Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–22.
Heald AH, Jenkins DA, Williams R, Sperrin M, Fachim H, Mudaliar RN, Syed A, Naseem A, Gibson JM, Bowden Davies KA, Peek N, Anderson SG, Peng Y, Ollier W. The Risk Factors potentially influencing hospital admission in people with diabetes, following SARS-CoV-2 infection: a population-level analysis. Diabetes Ther. 2022;13:1007–21.
Heald AH, Jenkins DA, Williams R, Sperrin M, Mudaliar RN, Syed A, Naseem A, Bowden Davies KA, Peng Y, Peek N, Ollier W, Anderson SG, Delanerolle G, Gibson JM. Mortality in people with type 2 diabetes following SARS-CoV-2 infection: a population level analysis of potential risk factors. Diabetes Ther. 2022;13:1037–51.
Hippisley-Cox J, Coupland CA, Mehta N, Keogh RH, Diaz-Ordaz K, Khunti K, Lyons RA, Kee F, Sheikh A, Rahman S, Valabhji J, Harrison EM, Sellen P, Haq N, Semple MG, Johnson PWM, Hayward A, Nguyen-Van-Tam JS. Risk prediction of COVID-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374: n2244.
Tian Z, Heald AH, Stedman M, Fachim H, Livingston M, Gibson M, Peng Y, Ollier W. Age of people with type 2 diabetes and the risk of dying following SARS-CoV-2 infection. Int J Clin Pract. 2021;75: e14053.
https://coronavirus.data.gov.uk/details/vaccinations: Accessed 12 August 2022
https://www.gov.uk/government/publications/covid-19-vaccination-programme-guidance-for-healthcare-practitioners: Accessed 12 August 2022
Tessier E, Rai Y, Clarke E, Lakhani A, Tsang C, Makwana A, Heard H, Rickeard T, Lakhani S, Roy P, Edelstein M, Ramsay M, Lopez-Bernal J, White J, Andrews N, Campbell CNJ, Stowe J. Characteristics associated with COVID-19 vaccine uptake among adults aged 50 years and above in England (8 December 2020–17 May 2021): a population-level observational study. BMJ Open. 2022;12: e055278.
Dolan SA, Jarman B, Bajekal M, Davies PM, Hart D. Measuring disadvantage: changes in the underprivileged area, Townsend, and Carstairs scores 1981–91. J Epidemiol Commun Health. 1995;49(Suppl 2):S30–3.
https://healthinnovationmanchester.com/thegmcarerecord/the-gm-care-record-for-secondary-uses-research: Accessed 21 May 2021
https://healthinnovationmanchester.com: Accessed 21 May 2021
Brainard J, Grossi Sampedro CM, Sweeting A, Fordham R. Was alpha deadlier than wild-type COVID? Analysis in rural England. Infection. 2022;5:1–8.
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6.
Hussain A, Boulton AJM. COVID-19 and diabetes: international diabetes federation perspectives. Diabetes Res Clin Pract. 2020;167: 108339.
Pal R, Bhadada SK, Misra A. COVID-19 vaccination in patients with diabetes mellitus: current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15:505–8.
American Diabetes Association 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43:S37–47.
Smith SA, Poland GA. Use of influenza and pneumococcal vaccines in people with diabetes. Diabetes Care. 2000;23:95–108.
Looijmans-Van den Akker I, Verheij TJM, Buskens E, Nichol KL, Rutten GEHM, Hak E. Clinical effectiveness of first and repeat influenza vaccination in adult and elderly diabetic patients. Diabetes Care. 2006;29:1771–6.
Stedman M, Davies M, Lunt M, Verma A, Anderson SG, Heald AH. A phased approach to unlocking during the COVID-19 pandemic-Lessons from trend analysis. Int J Clin Pract. 2020;74(8): e13528.
https://www.ons.gov.uk: Accessed 12 August 2022
Acknowledgements
The time of DJ, RW and NP was funded by the National Institute for Health Research (NIHR) Greater Manchester Patient Safety Translational Research Centre. The time of RW was partially funded by the NIHR Applied Research Collaboration Greater Manchester (NIHR200174). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Funding
The journal’s Rapid Service Fee was funded by the authors.
Author Contribution
AHH, DJ and RW led on the writing of this paper. DJ conducted the data analysis. KBD, JMG and WO provided input in relation to context and interpretation of the findings and contributed at all stages. RNJ and AN provided essential clinical context and inputted at all stages of conception and writing.
Disclosures
Adrian H Heald, David A Jenkins, Richard Williams, Rajshekhar N Mudaliar, Asma Naseem, Kelly A Bowden Davies, J Martin Gibson, Yonghong Peng and William Ollier have nothing to disclose.
Compliance with Ethics Guidelines
The project was approved and overseen by Health Innovation Manchester.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Heald, A.H., Jenkins, D.A., Williams, R. et al. COVID-19 Vaccination and Diabetes Mellitus: How Much Has It Made a Difference to Outcomes Following Confirmed COVID-19 Infection?. Diabetes Ther 14, 193–204 (2023). https://doi.org/10.1007/s13300-022-01338-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01338-5