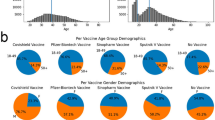
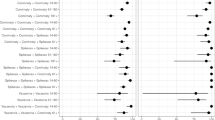
Abstract
Since SARS-CoV-2 was first identified as the cause of a cluster of cases of pneumonia in the Hubei Province in China in late 2019, the development and distribution of effective vaccines to prevent severe illness and death from the disease has been a major global priority. Because of the initial concerns about worse disease severity and prognosis in the setting of diabetes mellitus, people with diabetes were an early priority for these vaccines as they became available. The rapid development of vaccines is a remarkable story of collaboration in the scientific community and has become one of the most effective ways to mitigate the impact of the COVID-19 pandemic. This chapter explores what is known about the general approach to vaccination, the duration of vaccines, the impact vaccines have had on hospitalization and mortality in persons with diabetes and COVID-19, and strategies to improve vaccine confidence in patients living with diabetes.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–90.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Bashir S, Alabdulkarim N, Altwaijri N, Alhaidri N, Hashim R, Nasim E, et al. The battle against the COVID-19 pandemic—a perspective from Saudi Arabia. One Health. 2021;12:100229.
Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1):S27–36.
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8.
Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43(6):867–9.
Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403.
Huang Y, Yang C, Xu X, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacologica Sinica. 2020;3(41):1141–9.
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3.
Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–27.
Alqassieh R, Suleiman A, Abu-Halaweh S, Santarisi A, Shatnawi O, Shdaifat L, et al. Pfizer-BioNTech and Sinopharm: a comparative study on post-vaccination antibody titers. Vaccines (Basel). 2021;9(11):1223.
Lampasona V, Secchi M, Scavini M, Bazzigaluppi E, Brigatti C, Marzinotto I, et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020;63(12):2548–58.
Karamese M, Tutuncu EE. The effectiveness of inactivated SARS-CoV-2 vaccine (CoronaVac) on antibody response in participants aged 65 years and older. J Med Virol. 2022;94(1):173–7.
D’Addio F, Sabiu G, Usuelli V, Assi E, Abdelsalam A, Maestroni A, et al. Immunogenicity and safety of SARS-CoV-2 mRNA vaccines in a cohort of patients with type 1 diabetes. Diabetes. 2022;71(8):1800–6.
Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85.
Rosenberg ES, Holtgrave DR, Dorabawila V, Conroy M, Greene D, Lutterloh E, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status - New York, May 3-July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1150–5.
Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44.
Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med. 2022;386(4):340–50.
Yek C, Warner S, Wiltz JL, Sun J, Adjei S, Mancera A, et al. Risk Factors for severe COVID-19 outcomes among persons who completed primary COVID-19 vaccination series. MMWR Morb Mortal Wkly Rep. 2022;71(1):19–25.
Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):139–45.
Moreira ED Jr, Kitchin N, Xu X, Dychter SS, Lockhart S, Gurtman A, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022;386(20):1910–21.
CDC recommends the first updated COVID-19 booster; 1 Sep 2022. https://www.cdc.gov/media/releases/2022/s0901-covid-19-booster.html. Accessed 9 Sep 2022.
Interim clinical considerations for COVID-19 vaccines: bivalent boosters. ACIP; 1 Sep 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/09-covid-hall-508.pdf. Accessed 9 Sep 2022.
Rosenblum HG, Wallace M, Godfrey M, Roper LE, Hall E, Fleming-Dutra KE, et al. Interim recommendations from the advisory committee on immunization practices for the use of bivalent booster doses of COVID-19 vaccines—United States, October 2022. MMWR Morb Mortal Wkly Rep. 2022;71(45):1436–41.
Coronavirus (COVID-19) update: FDA authorizes updated (bivalent) covid-19 vaccines for children down to 6 months of age; 8 Dec 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-updated-bivalent-covid-19-vaccines-children-down-6-months. Accessed 29 Dec 2022.
Puig-Domingo M, Marazuela M, Yildiz BO, Giustina A. COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine. 2021;72(2):301–16.
Rao GHR. Twindemic of coronavirus disease (COVID-19) and cardiometabolic diseases. Int J Biomedicine. 2021;11(2):111–22.
Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–8.
Holman N, Knighton P, Kar P, O’Keefe J, Curley M, Weaver A, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–33.
Myers AK, Kim TS, Zhu X, Liu Y, Qiu M, Pekmezaris R. Predictors of mortality in a multiracial urban cohort of persons with type 2 diabetes and novel coronavirus 19. J Diabetes. 2021;13(5):430–8.
McGovern AP, Thomas NJ, Vollmer SJ, Hattersley AT, Mateen BA, Dennis JM. The disproportionate excess mortality risk of COVID-19 in younger people with diabetes warrants vaccination prioritization. Diabetologia. 2021;64(5):1184–6.
Powers AC, Aronoff DM, Eckel RH. COVID-19 vaccine prioritisation for type 1 and type 2 diabetes. Lancet Diabetes Endocrinol. 2021;9(3):140–1.
Gregory JM, Slaughter JC, Duffus SH, Smith TJ, LeStourgeon LM, Jaser SS, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526–32.
Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–22.
O’Malley G, Ebekozien O, Desimone M, Pinnaro CT, Roberts A, Polsky S, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D exchange multicenter surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936–42.
Wargny M, Gourdy P, Ludwig L, Seret-Bégué D, Bourron O, Darmon P, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174–7.
Afshar ZM, Babazadeh A, Janbakhsh A, Mansouri F, Sio TT, Sullman MJM, et al. Coronavirus disease 2019 (Covid-19) vaccination recommendations in special populations and patients with existing comorbidities. Rev Med Virol. 2021;22:e2309.
Dispinseri S, Lampasona V, Secchi M, Cara A, Bazzigaluppi E, Negri D, et al. Robust neutralizing antibodies to SARS-CoV-2 develop and persist in subjects with diabetes and COVID-19 pneumonia. J Clin Endocrinol Metab. 2021;106(5):1472–81.
Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, et al. The advisory Committee on Immunization practices’ updated interim recommendation for allocation of COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657–60.
World Health Organization. WHO SAGE roadmap for prioritizing use of COVID-19 vaccines; 21 Jan 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2022.1. Accessed 29 Dec 2022.
American Diabetes Association applauds CDC decision to prioritize all people with diabetes for the COVID-19 vaccine; 30 Mar 2021. https://diabetes.org/official-statements/2021/ADA-applauds-CDC-decision-to-prioritize-all-pwd-for-covid-19-vaccine. Accessed 29 Dec 2022.
Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, Committee of Clinical Practice Guidelines, Korean Diabetes Association, et al. 2021 clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021;45(4):461–81.
European Society of Endocrinology (ESE)’s statement concerning COVID-19 vaccination: ‘follow the same recommendations for patients with stable endocrine disorders as for the general population’; February 2021. https://www.ese-hormones.org/news/ese-news/european-society-of-endocrinology-ese-s-statement-concerning-covid-19-vaccination-follow-the-same-recommendations-for-patients-with-stable-endocrine-disorders-as-for-the-general-population/. Accessed 15 Feb 2022.
Sculli MA, Formoso G, Sciacca L. COVID-19 vaccination in pregnant and lactating diabetic women. Nutr Metab Cardiovasc Dis. 2021;31(7):2151–5.
Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1–236.e14.
World Health Organization. Ten threats to global health in 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019. Accessed 7 Sep 2022.
MacDonald NE and the SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4.
Marcelin JR, Swartz TH, Bernice F, Berthaud V, Christian R, da Costa C, et al. Addressing and inspiring vaccine confidence in Black, Indigenous and People of Color during the Coronavirus Disease 2019 pandemic. Open Forum Infect Dis. 2021;8(9):ofab417.
World Health Organization. Infodemic. https://www.who.int/health-topics/infodemic#tab=tab_1. Accessed 7 Sep 2022.
Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5(3):337–48.
Fridman A, Gershon R, Gneezy A. COVID-19 and vaccine hesitancy: a longitudinal study. PLoS One. 2021;16(4):e0250123.
Savoia E, Piltch-Loeb R, Goldberg B, Miller-Idriss C, Hughes B, Montrond A, et al. Predictors of COVID-19 vaccine hesitancy: socio-demographics, co-morbidity, and past experience of racial discrimination. Vaccines. 2021;9(7):767.
Hamel L, Lopes L, Muñana, C, Artiga S, Brodie M. Kaiser Family Foundation (KFF). Race, health and COVID-19: the views and experiences of Black Americans. Key findings from the KFF/undefeated survey on race and health. https://files.kff.org/attachment/Report-Race-Health-and-COVID-19-The-Views-and-Experiences-of-Black-Americans.pdf. Accessed 9 Sep 2022.
Painter JE, Viana De O, Mesquita S, Jimenez L, Avila AA, Sutter CJ, Sutter R. Vaccine-related attitudes and decision-making among uninsured, Latin American immigrant mothers of adolescent daughters: a qualitative study. Hum Vaccin Immunother. 2019;15(1):121–33.
Hildreth JEK, Alcendor DJ. Targeting COVID-19 Vaccine Hesitancy in Minority Populations in the US: Implications for Herd Immunity. Vaccines (Basel). 2021;9(5):489.
Gatwood J, McKnight M, Fiscus M, Hohmeier KC, Chisholm-Burns M. Factors influencing likelihood of COVID-19 vaccination: a survey of Tennessee adults. Am J Health Syst Pharm. 2021;78(10):879–89.
Scoccimarro D, Panichi L, Ragghianti B, et al. Sars-CoV2 vaccine hesitancy in Italy: a survey on subjects with diabetes. Nutr Metab Cardiovasc Dis. 2021;31(11):3243–6.
Al-Hanawi MK, Ahmad K, Haque R. Willingness to receive COVID-19 vaccination among adults with chronic diseases in the Kingdom of Saudi Arabia. J Infect Public Health. 2021;14(10):1489–96.
Bongomin F, Olum R, Andia-Biraro I, et al. COVID-19 vaccine acceptance among high-risk populations in Uganda. Ther Adv Infect Dis. 2021;9(8):20499361211024376.
Abedin M, Islam MA, Rahman FN, Reza HM, Hossain MZ, Hossain MA, et al. Willingness to vaccinate against COVID-19 among Bangladeshi adults: Understanding the strategies to optimize vaccination coverage. PLoS One. 2021;16(4):e0250495.
Al-Marshoudi S, Al-Balushi H, Al-Wahaibi A, et al. Knowledge, attitudes, and practices (Kap) toward the covid-19 vaccine in Oman: a pre-campaign cross-sectional study. Vaccines (Basel). 2021;9(6):602.
Umakanthan S, Patil S, Subramaniam N, et al. COVID-19 vaccine hesitancy and resistance in India explored through a population-based longitudinal survey. Vaccines (Basel). 2021;9(10):1064.
Syed Alwi SAR, Rafidah E, Zurraini A, Juslina O, Brohi IB, Lukas S. A survey on COVID-19 vaccine acceptance and concern among Malaysians. BMC Public Health. 2021;21(1):1129.
Rutten LJ, Zhu X, Leppin AL, Ridgeway JL, Swift MD, Griffin JM, et al. Evidence-based strategies for clinical organizations to address COVID-19 vaccine hesitancy. Mayo Clin Proc. 2021;96(3):699–707.
Bogart LM, Dong L, Gandhi P, Klein DJ, Smith TL, Ryan S, Ojikutu BO. COVID-19 vaccine intentions and mistrust in a National Sample of Black Americans. J Natl Med Assoc. 2022;113(6):599–611.
Chu J, Pink SL, Willer R. Religious identity cues increase vaccination intentions and trust in medical experts among American Christians. Proc Natl Acad Sci U S A. 2021;118(49):e2106481118.
Diament SM, Kaya A, Magenheim EB. Frames that matter: increasing the willingness to get the Covid-19 vaccines. Soc Sci Med. 2022;292:114562.
Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 2020;38(42):6500–7.
Centers for Disease Control. Talking with patients about COVID-19 vaccination. https://www.cdc.gov/vaccines/covid-19/hcp/engaging-patients.html. Accessed 9 Sep 2022.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Crow, M.T., Johnson, E.N. (2023). COVID-19 Vaccination in Persons with Diabetes: How to Approach Patients. In: Myers, A.K. (eds) Diabetes and COVID-19. Contemporary Endocrinology. Springer, Cham. https://doi.org/10.1007/978-3-031-28536-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-28536-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28535-6
Online ISBN: 978-3-031-28536-3
eBook Packages: MedicineMedicine (R0)