Abstract
Background
TRUST-CHN is a prospective, post-marketing safety study in patients with type 2 diabetes mellitus (T2DM) in China to evaluate the safety and effectiveness of dulaglutide in real-world clinical practice. We report here the study design and baseline characteristics of enrolled patients.
Methods
The study design was described, and baseline data were analyzed, including demographic characteristics, T2DM duration, comorbidities, dulaglutide treatment patterns, and concomitant medications.
Results
For the present analysis of this ongoing study, data were collected from January 2020 to November 2021. A total of 3313 patients were enrolled, of whom 3294 patients were included in the safety analysis. In total, 1047 patients had a prior history of dulaglutide use before being enrolled in the study. The mean (standard deviation [SD]) age of study subjects was 50.1 (13.2) years, 85.1% were aged < 65 years; 67.9% were male, and 35.9% had an education of university level or higher. Mean (SD) duration of T2DM was 6.4 (6.7) years. Baseline mean (SD) glycated hemoglobin was 8.8% (2.2%), and mean (SD) body mass index was 28.1 (4.1) kg/m2. A total of 2867 (87%) patients had at least one comorbidity, the most frequently reported of which were overweight/obesity (87.1%), hyperlipidemia (50.5%), hypertension (47.9%), diabetic neuropathy (18.9%), and coronary artery disease (15.7%). Almost all (99.7%) patients were treated with 1.5 mg dulaglutide; at baseline, 24.8% were treated with this medication as monotherapy and 75.2% in combination therapy with other medications, including metformin (42.3%), sodium glucose co-transporter2 inhibitor (26.7%), insulin (18.3%), α-glucosidase inhibitor (13.1%), sulfonylurea (5.3%), dipeptidyl peptidase 4 inhibitor (4.4%), glucagon-like peptide 1 receptor agonist (2.7%), and thiazolidinedione (2.4%).
Conclusion
The present analysis revealed real-world baseline characteristics of patients with T2DM in China who use dulaglutide enrolled in TRUST-CHN. These data will enable further exploration of the characteristics of patients with T2DM in China and provide an insight on the current use of dulaglutide in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The TRUST-CHN is a post-marketing, safety study which, following a request by the National Medical Product Administration of China, is investigating the safety and exploratory effectiveness of dulaglutide in patients with type 2 diabetes mellitus (T2DM) in real-world clinical practice in China. |
This is the first and currently largest observational study of dulaglutide as well as the largest observational study of glucagon-like peptide-1 GLP-1 receptor agonists in patients with T2DM in China. |
What was learned from the study? |
The study design and baseline characteristics of patients with T2DM treated with dulaglutide in the TRUST-CHN study are reported. |
Mean age was approximately 50 years; most patients were male, and mean disease duration was approximately 6 years. Common comorbidities were overweight/obesity, hyperlipidemia, hypertension, diabetic neuropathy, and coronary artery disease. |
Data from the present analysis provides an insight into the T2DM patient profile and current treatment patterns of dulaglutide in clinical practice in China. |
Introduction
The prevalence of diabetes in China has rapidly increased over the last 40 years [1], increasing to 10.6% in 2021 [2]. Type 2 diabetes mellitus (T2DM) seems to develop at a lower body mass index (BMI) in the Chinese population compared to the European population [3]. The Chinese Diabetes Society guidelines for T2DM recommend metformin as a first-line pharmacological treatment for T2DM [4]. When metformin monotherapy does not lead to glycemic control, it should be combined with one or two drugs with different mechanisms of action, followed by escalation to metformin plus multiple daily injections of premixed insulin [1].
Glucagon-like peptide-1 (GLP-1) is a gut-derived incretin hormone; GLP-1 receptor agonists (RAs) are injectable incretin mimetics that stimulate insulin, lower glucagon secretion, inhibit gastric emptying, and reduce appetite [5]. Dulaglutide is a long-acting human GLP-1 RA, with approximately 90% homology to endogenous GLP-1 [6, 7]. Long-term phase 3 trials in patients, the majority of whom were White, have shown that both dulaglutide monotherapy and dulaglutide as adjunct to metformin therapy provide superior glycemic control compared with metformin monotherapy and sitagliptin (dipeptidylpeptidase 4 [DPP4] inhibitor) monotherapy. Dulaglutide added to pioglitazone and metformin also showed superior glycemic control versus exenatide (GLP1 RA), with a tolerability profile consistent with that seen with other medications in the GLP-1 RA class [8,9,10]. In East Asian patients with T2DM, once-weekly dulaglutide monotherapy showed superior glycemic control compared with daily glimepiride [11]. In a population consisting mainly of Asian patients with T2DM who had previously failed to achieve optimal glycemic control on metformin and/or a sulfonylurea treatment, once-weekly dulaglutide resulted in a greater improvement in glycated hemoglobin (HbA1c) levels, combined with weight loss and less-pronounced hypoglycemia, compared with once-daily insulin glargine [12].
GLP-1 RAs have shown a benefit in cardiovascular outcomes in phase 3 cardiovascular outcome trials (CVOTs), including LEADER® (liraglutide; ClinicalTrials.gov Identifier: NCT01179048) [13], SUSTAIN™-6 (semaglutide; NCT01720446) [14], EXSCEL (exenatide; NCT01144338) [15], and REWIND (dulaglutide; NCT01394952) [16]. However, the proportions of Chinese or even Asian patients in the study populations of these CVOTs were limited; consequently, the generalizability of these results to Chinese patients was further investigated in the REWIND trial, using once-weekly dulaglutide; the results were shown to be the most generalizable to Chinese patients among the four former CVOTs [17].
Dulaglutide was approved in the USA, EU, and China as an adjunct to diet and exercise to improve glycemic control in adults with T2DM [18,19,20] and, specifically in the USA, to reduce the risk of major adverse cardiovascular events in adults with T2DM who have cardiovascular disease or cardiovascular risk factors [19]. In China, dulaglutide is approved either as monotherapy for patients with T2DM with poor glycemic control (as an adjunct to diet and exercise) or as combination therapy for patients with poor glycemic control after metformin, or sulfonylureas, or metformin combined with sulfonylureas [20].
At the time of dulaglutide approval in China, and as per the National Medical Product Administration request, a post-marketing safety study (TRUST-CHN) was initiated to further investigate the safety of dulaglutide use in Chinese patients with T2DM. The TRUST-CHN study is a prospective, observational study designed to collect safety data from the real-world prescribing practice in a large cohort of patients with T2DM in China. The exploratory objectives are to evaluate the effectiveness and treatment satisfaction of dulaglutide in real-world clinical practice. Here, we describe the study design together with the baseline characteristics of Chinese patients with T2DM who are enrolled in the TRUST-CHN study.
Methods
The primary outcomes of the TRUST-CHN study are treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs) in patients with T2DM treated with dulaglutide over a 24-week period. Exploratory objectives include evaluation of the effectiveness of dulaglutide and treatment satisfaction after 24 weeks of treatment. The study was ongoing at the time of the present analyses, in which we describe patients’ baseline characteristics. The data were collected from January 2020 to November 2021 (cutoff September 2021 and programmed November 2021).
Study Design and Study Population
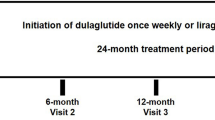
This is a single-country, prospective, non-interventional, safety study to collect and estimate the incidence of TEAEs, SAEs, and adverse drug reactions) in patients in China with T2DM receiving treatment with dulaglutide over a 24-week period. The present analysis is the first readout of the entire baseline data. Patients had three scheduled visits, at baseline (Visit 1), after 4 weeks (Visit 2), and after 24 weeks (Visit 3) of treatment (see Fig. 1).
As exploratory objectives (data not presented here), the effectiveness of dulaglutide will also be evaluated by collecting data on HbA1c and body weight, as well as on treatment satisfaction using the Diabetes Treatment Satisfaction Questionnaire (DTSQ) [21, 22], after 24 weeks of treatment.
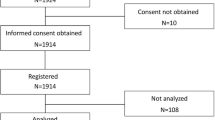
Patients who were aged ≥ 18 years, diagnosed with T2DM, prescribed dulaglutide according to the Chinese label, and who provided written consent for the release of their data were included in the study. Patients contraindicated for dulaglutide use or simultaneously participating in a different study with a treatment intervention, and patients who were pregnant, breastfeeding, or intending to become pregnant during the study were excluded.
The safety analysis population included all patients who took at least one dose of dulaglutide. This population was used for the primary endpoints and baseline analyses. The effectiveness analysis population included all patients who took at least one dose of dulaglutide and participated in at least one post-baseline follow-up visit within the defined visit window, i.e., those for whom baseline and any post-baseline observation data were available. The effectiveness analysis population was used for the exploratory endpoints.
Dulaglutide-naïve patients were defined as those who had never treated been with dulaglutide prior to study entry. Dulaglutide-experienced patients were those who had already been treated with dulaglutide prior to study entry, whether or not they were still receiving dulaglutide as treatment.
Treatment Setting
Treatment was prescribed in accordance with the dulaglutide (Trulicity®; Eli Lilly and Company, Indianapolis, IN, USA) prescribing information in China and the investigator’s/physician’s judgement. During dulaglutide treatment, blood glucose self-monitoring was not required; however, blood glucose self-monitoring may be required to adjust the sulfonylurea dose [20].
Outcomes
Patients will be observed for approximately 24 weeks following enrollment, with a total of three visits during the study, to enable data collection on patient demographics, patient-reported HbA1c, body weight, medical history, dulaglutide treatment patterns, concomitant medications, adverse events, and reasons for discontinuation (see Electronic Supplementary Material [ESM] Appendix 1: Table S1).
Only baseline data are presented in the current study, including demographic characteristics, T2DM disease history, comorbidities present at baseline, dulaglutide treatment history, other T2DM treatment history, concomitant T2DM medications, dulaglutide prescription patterns, HbA1c levels, body weight measurements, vital signs, and laboratory measurements, with a cutoff date of 8 November 2021.
Statistical Analyses
Demographic characteristics, duration of T2DM, comorbidities, dulaglutide treatment patterns, and concomitant medications were summarized using descriptive statistics.
The results of the analyses of categorical variables are presented as the frequency and percentage (with the percentage excluding the number missing in the denominator), and those of continuous variables are presented as the mean and standard deviation (SD). The disposition of all patients who entered the study was summarized. Statistical analysis was performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA).
Compliance with Ethics
This non-interventional, post-marketing safety study was submitted to ethical review boards for approval whenever required by local law. Regardless of local law, all primary data collection observational studies were submitted to at least one independent body for review and to confirm that the study is non-interventional. Additional information on the Ethics Review Board is provided in ESM Appendix 2.
Regulatory authorities were notified, and approval was sought as required by local laws and regulations. Progress reports were submitted to Ethics Review Boards and regulatory authorities as required by local laws and regulations. This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with good clinical practices and applicable laws and regulations of China.
Results
Baseline Analyses
Patient Disposition and Demographic Characteristics
Data from January 2020 to November 2021 were analyzed. A total of 3313 patients were enrolled in the study; of these, four were inadvertently enrolled who were subsequently deemed not eligible, due to inclusion/exclusion criteria, and excluded. Of the eligible patients, 3294 were included in the safety analysis population. Of these, 1047 were dulaglutide-experienced and 2247 were dulaglutide-naïve. In the effectiveness analysis population, 3201 patients were included; of these, 1028 were dulaglutide-experienced and 2173 were dulaglutide-naïve.
Demographic characteristics, including age, sex, and education level, are presented in Table 1. The mean age of the population was approximately 50 years, with 2803 (85.1%) patients aged < 65 years. The majority of patients were male (2236; 67.9%) and 1182 (35.9%) of patients had an educational level of university level or higher.
Baseline HbA1c Levels, Body Weight, and BMI
Mean HbA1c levels at baseline were 8.8%, and mean baseline body weight was 80.2 kg. Body weight and HbA1c levels in the effectiveness analysis population are summarized in Table 2.
In the safety analysis population, based on the global BMI classification, 52.0% of patients were overweight and 27.1% were obese at baseline. However, based on the Chinese BMI classification [23], 41.4% of patients were overweight and 45.7% were obese. Data on baseline BMI, waistline, hipline, and waist-to-hip ratio by dulaglutide treatment experience were analyzed further using the safety analysis population and is summarized in Table 2.
Medical History and Comorbidities
Data on T2DM disease history, including age at diagnosis and disease duration, are presented in Table 3. The mean age at T2DM diagnosis was approximately 43 years, with a mean disease duration of 6.4 years.
Of the 3294 patients in the safety analysis population, 2867 (87.0%) had at least one existing medical condition at baseline. Comorbidities reported in ≥ 5% of the total safety analysis population are presented in Table 3. The most frequently reported (≥ 15% patients) comorbidities were overweight/obesity, hyperlipidemia, hypertension, diabetic neuropathy, and coronary artery disease.
Concomitant T2DM Medications at Baseline
In the safety analysis population, 2178 (66.1%) patients were being treated with at least one concomitant medication for T2DM at baseline; the most commonly reported medications were metformin (42.3%) and sodium glucose co-transporter-2 (SGLT2) inhibitor (26.7%). The types of concomitant medications are summarized in Table 4. The history of other T2DM treatments is summarized in ESM Appendix 1: Table S2.
Dulaglutide Prescription Patterns
In the safety analysis population, 816 (24.8%) patients received dulaglutide monotherapy; 2478 (75.2%) received combination therapy at baseline. Data on the dosage and frequency of dulaglutide administration are summarized in Table 5. Dulaglutide treatment history is summarized in ESM Appendix 1: Table S3.
Baseline Vital Signs
At baseline, 60.3% patients had a systolic blood pressure in the range of 120–140 mmHg, and 43.4% had a diastolic blood pressure in the range of 80–90 mmHg. Mean systolic and diastolic blood pressure was 130.7 and 81.6 mmHg, respectively. Systolic blood pressure, diastolic blood pressure, and heart rate at baseline according to dulaglutide treatment experience are summarized in ESM Appendix 1: Table S4.
Baseline Laboratory Measurements
At baseline, among patients whose laboratory measurements were taken, 71.9% (612/851) had fasting insulin levels within the normal limit, with a mean value of 20.0 mU/L. Fasting blood glucose was above the upper normal limit in 80.9% (954/1179) of patients, with a mean value of 9.1 mmol/L. Two hours post-prandial blood glucose was above the upper normal limit in 79.1% (566/716) of patients, with a mean value of 15.5 mmol/L. Laboratory measurements at baseline according to dulaglutide treatment experience are further summarized in ESM Appendix 1: Table S5.
Discussion
The TRUST-CHN study is the first and largest observational study of dulaglutide conducted to date, as well as the largest observational study of GLP-1 RA in patients with T2DM in China. Baseline data from this study provide an insight into the current use of dulaglutide in real-world clinical practice, the demographic characteristics of patients with T2DM outside of the clinical trial setting, and the medical history of these patients. Among the 3294 patients included in the safety analysis set, the mean age was approximately 50 years, the majority of patients (67.9%) were male, and approximately one-third of patients had an educational background of university level or higher (35.9%).
The mean patient age at T2DM diagnosis was approximately 43 years, with a mean disease duration of 6.4 years at this time. Mean HbA1c levels were 8.8% and 8.9% in the dulaglutide-experienced and dulaglutide-naïve patients, respectively. The mean BMI was 28.1 kg/m2; based on the Chinese BMI classification, 45.7% of patients were obese and 41.4% were overweight and, therefore, 87.1% of the patients were overweight or had obesity. In comparison, based on the global BMI classification, 27.1% of patients were in the obesity category and 52.0% were in the overweight category.
Most patients (87%) had at least one existing comorbid condition at baseline, with the most frequent comorbidities being overweight/obesity (87.1%), hyperlipidemia (50.5%), hypertension (47.9%), diabetic neuropathy (18.9%), and coronary artery disease (15.7%).
Among the 3294 patients included in the safety analysis set, 1047 had already received dulaglutide prior to this being enrolled in this study. In total, 66.1% of patients were on at least one concomitant medication for T2DM at baseline, most commonly metformin (42.3%) and SGLT-2 inhibitors (26.7%). These observations suggest that metformin remains one of the most common T2DM medications, followed by SGLT-2 inhibitors. In total, 33.1% and 21.3% patients were on one and two oral anti-diabetic medications, respectively, which suggests that concomitant use of oral anti-diabetic drugs is common in clinical practice.
The Chinese Diabetes Society guidelines recommend that when patients cannot achieve the blood glucose target on metformin monotherapy, they should be put on dual therapy with insulin secretagogues, α-glucosidase inhibitors, DPP4 inhibitors, thiazolidinedione, SGLT-2 inhibitors, or GLP-1 RAs [1], with the last typically initiated when patients no longer achieve glycemic control with one or more oral anti-hyperglycemic drugs [1, 24]. Another challenge in the treatment of patients with T2DM is the control of risk factors for cardiovascular disease [3]. Both the ADA and EASD recommend the combination of metformin with either a SGLT-2 inhibitor or a GLP-1 RA for T2DM patients with atherosclerotic cardiovascular disease, with proven cardiovascular benefit [24].
Dulaglutide has shown reduced cardiovascular outcomes in T2DM patients with or without previous cardiovascular disease, with an effect size similar to that observed with other GLP-1 RAs CVOTs [16]. The REWIND trial enrolled 9901 patients with T2DM who had either a previous cardiovascular event or a cardiovascular risk factor. Of these, 4949 were treated with dulaglutide; 53.4% of patients were male, 75.9% were White, mean age was 66.2 years, and mean T2DM disease duration was 10.5 years [16].
In a subgroup analysis of the AWARD-CHN2 trial, once-weekly dulaglutide was compared with once-daily insulin glargine in a Chinese T2DM population, of whom 60.9% were male, the mean age was 54.5 years, the mean diabetes duration was 7.9 years, and the mean BMI was 26.0 kg/m2 [25]. Once weekly-dulaglutide was shown to be superior to once-daily glargine, with more pronounced reductions in body weight and hypoglycemia rates in Chinese patients with T2DM who had inadequate glycemic control with one to two oral anti-hyperglycemic medications [25]. The baseline characteristics of the TRUST-CHN study presented here are similar to those of this subgroup analysis, with a numerically higher percentage of male patients (67.9%), slightly younger mean age (50 years), and a higher mean BMI (28.1 kg/m2).
In a post-hoc analysis of two Chinese patient populations with T2DM, the efficacy of dulaglutide was further evaluated according to baseline HbA1c < 8.5% or ≥ 8.5% after 26 weeks of treatment [26]. Dulaglutide achieved a greater HbA1c reduction, combined with weight loss and lower risk of hypoglycemia, compared with active comparators (glimepiride and insulin glargine) regardless of baseline HbA1c; patients with a higher baseline HbA1c did achieve greater HbA1c reductions [26]. In the same Chinese populations, another post-hoc analysis showed that dulaglutide improved glycemic control combined with a slight body weight reduction and low hypoglycemia risk in patients with a BMI < 25 kg/m2; this result suggests that BMI is not a necessary consideration for dulaglutide treatment in Chinese patients with T2DM [27]. In the present study, baseline HbA1c levels were patient-reported, with a mean value of 8.8% and a mean BMI of 28.1 kg/m2; these values indicate that Chinese diabetologists tend to prescribe dulaglutide for relatively obese patients or for patients with higher HbA1c in real-world clinical practice.
In a cross-sectional, observational study of 25,817 patients with T2DM in China, comorbid hypertension and/or dyslipidemia were seen in 72% of patients [28]. This is a trend that was also seen in the baseline characteristics of the present study, with hypertension and hyperlipidemia being the two most frequently reported comorbidities, occurring in 47.9% and 50.5% of patients, respectively. A recent review of real-world data from 29 studies across Canada, Europe, India, Japan, Malaysia, the Republic of Korea, and the USA showed dulaglutide to be beneficial in these populations [29]. However, more real-world data, specifically in Chinese patients, are needed to further elucidate the use and benefit of dulaglutide in patients with T2DM in China.
While the current study is designed to provide real-world data, it does have several limitations. The study duration was relatively short and HbA1c level was patient-reported. As this was a real-world sample, patients were included regardless of disease severity, and any factors that might have led to the decision of using dulaglutide versus alternative medications are not known.
Conclusions
To our knowledge, this is the first and largest observational study to date in patients with T2DM in China, who received dulaglutide either as monotherapy or in combination with other T2DM treatments.
Real-world data and evidence will help further advance knowledge on the benefit of dulaglutide in clinical practice and will enable further studies on the characteristics of patients with T2DM in China that may shape future, more individualized treatment algorithms.
References
Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. https://doi.org/10.1002/dmrr.3158.
International Diabetes Federation. IDF atlas, 10th edition. China—diabetes report 2000–2045. 2021. Brussels: IDF. https://diabetesatlas.org/data/en/country/42/cn.html. Accessed 3 Dec 2021
Hu C, Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes. 2018;67(1):3–11. https://doi.org/10.2337/dbi17-0013.
Diabetes Association of Chinese Medical Association. Guideline for prevention and treatment of type 2 diabetes in China (2020 Edition). Chin Diabetes J. 2021;13(4):315–409. https://doi.org/10.3760/cma.j.cn115791-20210221-00095.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705. https://doi.org/10.1016/S0140-6736(06)69705-5.
Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26(4):287–96. https://doi.org/10.1002/dmrr.1080.
Sharma D, Verma S, Vaidy S, Kalia K, Tiwari V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed Pharmacother. 2018;108:952–62. https://doi.org/10.1016/j.biopha.2018.08.088.
Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–58. https://doi.org/10.2337/dc13-2761.
Umpierrez G, Povedano ST, Manghi FP, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168–76. https://doi.org/10.2337/dc13-2759.
Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159–67. https://doi.org/10.2337/dc13-2760.
Chen YH, Huang C-N, Cho Y-M, et al. Efficacy and safety of dulaglutide monotherapy compared with glimepiride in East-Asian patients with type 2 diabetes in a multicentre, double-blind, randomized, parallel-arm, active comparator, phase III trial. Diabetes Obes Metab. 2018;20(9):2121–30. https://doi.org/10.1111/dom.13340.
Wang W, Nevárez L, Filippova E, et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: a 52-week open-label, randomized phase III trial. Diabetes Obes Metab. 2019;21(2):234–43. https://doi.org/10.1111/dom.13506.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. https://doi.org/10.1056/NEJMoa1607141.
Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. https://doi.org/10.1056/NEJMoa1612917.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. https://doi.org/10.1016/S0140-6736(19)31149-3.
Cai X, Ji L. Generalizability of the results of cardiovascular outcome trials of glucagon-like peptide 1 receptor agonists in chinese patients with type 2 diabetes mellitus. Diabetes Ther. 2021;12(7):1861–70. https://doi.org/10.1007/s13300-021-01079-x.
European Medical Agency. Trulicity (dulaglutide) solution for injection. Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/trulicity-epar-product-information_en.pdf. Last updated Aug 2019. Accessed 06 Dec 2021.
Eli Lilly and Company. Trulicity (dulaglutide) injection, for subcutaneous use. Prescribing information. https://pi.lilly.com/us/trulicity-uspi.pdf. Last updated Dec 2021. Accessed 06 Dec 2021.
Eli Lilly and Company. Trulicity (dulaglutide) Prescribing information China. https://www.lillymedical.cn/zh-cn/diabetes/trulicity. Last updated Jan 2021. Accessed 22 Feb 2022.
Bradley C. Diabetes treatment satisfaction questionnaire (DTSQ). In: Bradley C, editor. Handbook of psychology and diabetes: a guide to psychological measurement in diabetes research and practice. Hove and New York: Routledge; 1994. p. 111–32.
Bradley C, Gamsu DS. Guidelines for encouraging psychological well-being: report of a Working Group of the World Health Organization Regional Office for Europe and International Diabetes Federation European Region St Vincent Declaration Action Programme for Diabetes. Diabet Med. 1994;11(5):510–6. https://doi.org/10.1111/j.1464-5491.1994.tb00316.x.
Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701. https://doi.org/10.2337/dci18-0033.
Li Y, Li L, Peng YD, et al. Efficacy and safety of dulaglutide versus insulin glargine in Chinese T2DM Patients: a subgroup analysis of a randomized trial (AWARD-CHN2). Diabetes Ther. 2019;10(4):1435–52. https://doi.org/10.1007/s13300-019-0646-y.
Yu M, Yuan GY, Zhang B, Wu HY, Lv XF. Efficacy and safety of dulaglutide by baseline HbA1c in Chinese Patients with type 2 diabetes: a post hoc analysis. Diabetes Ther. 2020;11(5):1147–59. https://doi.org/10.1007/s13300-020-00804-2.
Shi Y, Liu S, Zhu J, Hong T. Efficacy and safety of once-weekly dulaglutide in adult Chinese patients with type 2 diabetes and lower baseline body mass index. J Diabetes. 2021;13(4):353–7. https://doi.org/10.1111/1753-0407.13147.
Ji L, Hu D, Pan C, et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med. 2013;126(10):925 e11-22. https://doi.org/10.1016/j.amjmed.2013.02.035.
Robinson S, Boye KS, Mody R, et al. Real-world effectiveness of dulaglutide in patients with type 2 diabetes mellitus: a literature review. Diabetes Ther. 2020;11(7):1437–66. https://doi.org/10.1007/s13300-020-00839-5.
Acknowledgements
The authors would like to thank all patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This post-marketing safety study was funded by Eli Lilly and Company. Eli Lilly and Company provided funding for medical writing, editorial support, and publication fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Lixin Guo, Li Li, Qiurong Yu, Na Wang, Jun Chen, Zhiquan Wang, and Yuchen Ding. All authors contributed to the first draft and reviewed and commented on revisions until this manuscript was finalized. All authors read and approved the final manuscript.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Sharon Suntag, Chrysi Petraki, and Andrew McCulloch of IQVIA. Support for this assistance was funded by Eli Lilly and Company. The authors would like to thank Yihua Wang (MD, Eli Lilly and Company), Xiao Ma (PhD, Eli Lilly and Company), Yuying Deng (PhD, Eli Lilly and Company), Jinnan Li (MPH, Eli Lilly and Company) for review and critical suggestions for improvement.
Disclosures
Zhiquan Wang and Yuchen Ding are employees of and hold equity in Eli Lilly and Company. Lixin Guo, Li Li, Qiurong Yu, Na Wang, and Jun Chen declare that they have nothing to disclose.
Compliance with Ethics Guidelines
This non-interventional, post-marketing safety study was submitted to ethical review boards for approval whenever required by local law. Regardless of local law, all primary data collection observational studies were submitted to at least one independent body for review and to confirm that the study is considered non-interventional. Additional information on the Ethics Review Board is provided in ESM Appendix 2. Regulatory authorities were notified, and approval was sought as required by local laws and regulations. This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki (1964 and its later amendments) and that are consistent with good clinical practices and applicable laws and regulations of China.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Guo, L., Li, L., Yu, Q. et al. Study Design and Baseline Characteristics of Patients with T2DM in the Post-marketing Safety Study of Dulaglutide in China (TRUST-CHN). Diabetes Ther 13, 1231–1244 (2022). https://doi.org/10.1007/s13300-022-01268-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01268-2