Abstract
Introduction
iGlarLixi is indicated as an adjunct to diet and exercise in addition to metformin (with or without sodium-glucose cotransporter-2 inhibitors) to improve glycemic control in adults with insufficiently controlled type 2 diabetes (T2D). A cost-effectiveness analysis was conducted to compare iGlarLixi with premix biphasic insulin aspart 30 (BIAsp 30) in people with T2D suboptimally controlled with basal insulin (BI).
Methods
The IQVIA CORE Diabetes Model was used to estimate lifetime costs and outcomes for people with T2D from a UK health care perspective at a willingness-to-pay threshold of £20,000. Initial clinical data were based on the phase 3 randomized, open-label, active-controlled SoliMix clinical trial which compared the efficacy and safety of once-daily iGlarLixi with that of twice-daily BIAsp 30. Costs associated with management and complications and utilities values were derived from published sources. Lifetime costs (in £GBP) and quality-adjusted life-years (QALYs) were predicted; extensive scenario and sensitivity analyses were conducted.
Results
Estimated QALYs gained were slightly higher with iGlarLixi (8.9 vs. 8.8) compared with premix BIAsp 30, at a higher cost (£23,204 vs. £21,961). The base case incremental cost-effectiveness ratio (ICER) per QALY was £13,598. Treatment acquisition was the main driver of cost differences (iGlarLixi, £11,750; premix BIAsp 30, £10,395). Costs associated with management and complications were generally similar between comparators.
Conclusion
iGlarLixi provides improved QALY outcomes at an acceptable cost compared with premix BIAsp 30, with an ICER below the threshold generally considered acceptable by UK authorities. In people with T2D, iGlarLixi is a simple, cost-effective option for advancing therapy of BI, with fewer daily injections than premix BIAsp 30.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Premix insulins, including biphasic insulin aspart 30 (BIAsp 30), are widely used in people with type 2 diabetes (T2D) who require advancement of therapy but who are associated with increased risks of hypoglycemia and weight gain, compared with basal insulin (BI) plus glucagon-like peptide-1 receptor agonists, including the fixed-ratio combination of insulin glargine plus lixisenatide (iGlarLixi). |
The randomized phase 3 SoliMix trial demonstrated the efficacy and safety of once-daily iGlarLixi compared with twice-daily premix BIAsp 30 in people with T2D suboptimally controlled on BI. |
No economic comparison of iGlarLixi versus BIAsp 30 in the post-BI setting currently exists; the aim of this analysis was to compare the cost-effectiveness of iGlarLixi versus BIAsp 30 in people suboptimally controlled with BI in the context of the UK National Health System. |
What was learned from this study? |
Estimated quality-adjusted life-years (QALYs) gained were slightly higher with iGlarLixi versus premix BIAsp 30 (8.9 vs. 8.8), at a higher cost (£23,204 vs. £21,961); the base case incremental cost-effectiveness ratio per QALY was £13,598. |
In people living with T2D with suboptimal glycemic control during BI therapy, iGlarLixi confers slightly improved QALY outcomes at an acceptable cost compared with premix BIAsp 30. |
Introduction
It is estimated that approximately 3.6 million people are at increased risk of developing type 2 diabetes (T2D) in the UK, and the prevalence of people living with T2D is expected to increase to 5.5 million by 2030 [1]. A substantial proportion of people living with T2D experience suboptimal glycemic control during treatment with basal insulin (BI) analogs and often require dual or triple therapy [2, 3]. Guidelines from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend four approaches for advancement of therapy, including the addition of a rapid-acting insulin, multiple daily premix insulin doses (basal and prandial insulin co-formulation), or addition of daily or weekly glucagon-like peptide-1 receptor agonist (GLP-1 RA) to an existing BI regimen, or switching to a once-daily fixed-ratio combination (FRC) of BI and GLP-1 RA [2, 3].
Premix insulins (basal and prandial insulin co-formulation) are widely used in people who require advancement of therapy, accounting for around 30–36% of people living with T2D taking insulin globally. However, premix insulin is associated with an increased risk of hypoglycemia and weight gain compared with BI plus GLP-1 RAs [3,4,5,6]. Additionally, premix insulin requires multiple daily injections and frequent glucose monitoring, which may increase treatment burden and reduce adherence [7,8,9,10]. The phase 3 randomized, open-label, active-controlled SoliMix clinical trial compared the efficacy and safety of the once-daily FRC insulin glargine plus lixisenatide (iGlarLixi) with twice-daily premix biphasic insulin aspart 30 (BIAsp 30) in people living with T2D suboptimally controlled on BI combined with one or two oral anti-diabetes drugs (OADs; metformin with or without sodium-glucose cotransporter-2 [SGLT2] inhibitors). Once-daily iGlarLixi provided better glycemic control with weight benefit and less hypoglycemia than premix BIAsp 30 [11].
Insulin glargine 100 units/mL plus lixisenatide (iGlarLixi) is a combination of a long-acting human insulin analog with a GLP-1 RA that was initially approved in 2016 in the USA and in 2017 in Europe. It is indicated as an adjunct to diet and exercise in addition to metformin (with or without SGLT2 inhibitors) to improve glycemic control in adults with insufficiently controlled T2D [12]. Given the differences in clinical and cost profiles between therapies, cost-effectiveness analyses inform resource allocation within the budget constraints of health care systems. The aim of this analysis was to compare the cost-effectiveness of iGlarLixi versus BIAsp 30 in people with T2D suboptimally controlled with BI in the context of the UK National Health Service (NHS).
Methods
Study Overview
Cost-effectiveness for iGlarLixi versus premix BIAsp 30 was estimated using version 9.5 of the IQVIA CORE Diabetes Model (CDM). The IQVIA CDM is a non–product-specific computer simulation tool that models the effect of glucose monitoring, diabetes therapies, screening, and treatment strategies on the long-term health and economic outcomes of people living with type 1 and type 2 diabetes. The CDM uses a series of interdependent Markov submodels incorporating time-, state-, and diabetes type-dependent probabilities to simulate progression of disease-related complications using a set of equations for progression of the disease risk factors (United Kingdom Prospective Diabetes Study [UKPDS] Outcomes Model no. 68 [UKPDS 68]) [13] and for predicting the cardiovascular and mortality risk (UKPDS 82) [14]. The IQVIA CDM has been extensively validated and is widely used in diabetes research [15, 16]. The cost-effectiveness analysis reported here was conducted from the perspective of the UK NHS, assuming a willingness-to-pay (WTP) threshold of £20,000 per quality-adjusted life-year (QALY) gained. A hypothetical cohort of 1000 people was used, with a lifetime time horizon, and an annual discount rate of 3.5% for costs and outcomes in line with UK National Institute of Health and Care Excellence Decision Support Unit guidance [17]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Model Inputs and Structure
Baseline characteristics were primarily based on those reported in the SoliMix clinical trial (Table 1) [11]. For values not collected in the SoliMix trial (i.e., lipid parameters), data were extracted from the LixiLan-L trial and applied as a proxy for the population of interest [18]. For the other missing values, the CDM default values (population averages based on published literature) were used.
Efficacy data for iGlarLixi and premix BIAsp 30 from the SoliMix trial were used to predict glycated hemoglobin (HbA1c), body mass index (BMI), and hypoglycemia in the first year of the model (Table 2) [11]. After 1 year of treatment, the progression of HbA1c levels and other physiological parameters were predicted by the UKPDS 68 risk equation [13]. Simulated individuals were assumed to receive either iGlarLixi or premix BIAsp 30 until HbA1c returned to SoliMix trial baseline values (8.6%); at this point, individuals were assumed to switch to rescue therapy (consisting of basal plus rapid-acting insulin). HbA1c reductions during rescue therapy were sourced from the GetGoal Duo-2 trial, which reported HbA1c reductions of 0.6% when a prandial insulin bolus was added to BI therapy (with concomitant OADs). The use of iGlarLixi was assumed to result in a BMI decrease, whereas the use of BIAsp 30 was assumed to result in a BMI increase. When individuals switched to rescue therapy, BMI was assumed to increase based on observations from the GetGoal Duo-2 study. In addition, hypoglycemia rates in individuals who switched to rescue therapy were based on observations from the GetGoal Duo-2 study [19].
For the derivation of QALYs, utility values for health-related quality of life were obtained from published literature (Electronic Supplementary Material [ESM] Table S1) [20]. QALYs were assessed using the additive “Core Default Method,” in which current utility was based on the lowest-state utility of all concurrent comorbidities, and subsequent disutilities for complication events occurring in that year were applied; this results in an annual utility score for each simulated individual living with T2D.
Cost Data
Direct medical costs, comprising pharmacy costs, management costs (glucose test, needles, concomitant medication), and costs of T2D complications (cardiovascular disease complications, renal complications, acute events, eye disease, neuropathy, foot ulcer, amputation [ESM Table S2]) were calculated (all sources were converted to £2021). Unit costs were collected from published literature and UK national sources (Table 3). Per the SoliMix study design, self-monitoring blood glucose was assumed to occur once daily for individuals on iGlarLixi and twice daily for those on premix BIAsp 30; all patients were assumed to be also receiving metformin as concurrent oral diabetes therapy [11, 21].
In Europe, iGlarLixi is available as two formulations: 100 units/mL insulin glargine plus 50 µ/mL lixisenatide (Suliqua® SoloStar pen 10–40 units) and 100 units/mL insulin glargine plus 33 µ/mL lixisenatide (Suliqua® SoloStar pen 30–60 units [iGlarLixi 100/33]). A weighted average of the two formulations based on average daily dosing in the SoliMix trial was calculated for the first year. From the second year onward, it was assumed that only the iGlarLixi 100/33 formulation was used for the maintenance phase, considering the end-of-trial dose (40 units).
Analyses
Incremental differences in costs and QALYs were obtained for iGlarLixi versus premix BIAsp 30; incremental cost-effectiveness ratio (ICER) estimates for iGlarLixi relative to premix BIAsp 30 were calculated as the cost differential divided by the difference in QALYs and reported as costs per QALY. Scenario analyses were performed on key parameters to assess the robustness of the base case findings (ESM Table S3). A probabilistic sensitivity analysis (PSA) was also conducted to test uncertainty in the model by random variation of key parameter inputs within plausible distributions. Probabilistic distribution of key transition probabilities (myocardial infarction, stroke, congestive heart failure, angina) were applied by bootstrap sampling around the 95% confidence interval of the regression coefficient. For utilities and treatment effects, mean and standard error values were used to generate random sampling within a beta-distribution function. Direct costs (excluding acquisition costs, which were assumed to be fixed) were randomly sampled based on log-normal distribution within a 20% variance.
Results
Base Case Analysis
In the base case analysis, iGlarLixi was associated with a slightly higher QALY gain over the model time horizon (8.9 vs. 8.8; Table 4). Costs were slightly higher with iGlarLixi (£23,204 vs. £21,961), resulting in an ICER of £13,598 per QALY. Event rates for key diabetes-related complications were comparable for both treatment arms (ESM Fig. S1). Treatment switch to rescue therapy happened after year 6 in the iGlarLixi arm and after year 5 in the BIAsp 30 arm. In addition, treatment with iGlarLixi was associated with an initial decline in BMI, while patients receiving BIAsp 30 had an initial increase in BMI and a further increase when switched to rescue therapy (ESM Fig. S2). Cumulative incidence per 1000 patient-years of any hypoglycemic event was lower for iGlarLixi (40.42) compared with premix BIAsp 30 (43.27) and was similar for severe hypoglycemia incidence (0.12 vs. 0.13, respectively). A breakdown of the costs indicated that treatment acquisition was the main cost driver for both iGlarLixi (£11,750) and premix BIAsp 30 (£10,395; ESM Table S4). Costs associated with management and complications were generally similar between comparators.
Scenario Analyses
The robustness of the base case results was confirmed by extensive scenario analyses. iGlarLixi was shown to be a cost-effective alternative to BIAsp 30 in all scenarios tested, with all ICER estimates less than the WTP threshold of £20,000 per QALY (ESM Table S5).
Probabilistic Sensitivity Analysis
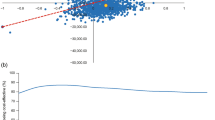
In the PSA analysis, 67% of iterations fell in the northeast quadrant of the cost-effectiveness plane, indicating that iGlarLixi was associated with an increase in QALY gained versus BIAsp 30, at a higher cost (Fig. 1a). At a WTP threshold of £20,000–30,000 per QALY gained, iGlarLixi was cost-effective in ~ 55–61% of cases (Fig. 1b).
Discussion
In this study, iGlarLixi was associated with slightly more QALYs gained versus premix BIAsp 30 in people with T2D suboptimally controlled with BI. The ICER for iGlarLixi was below the accepted WTP threshold of £20,000 per QALY gained, demonstrating that iGlarLixi is a cost-effective alternative to BIAsp 30 in this population; extensive scenario and sensitivity analyses confirmed the robustness of the base case findings. Although the unit cost of iGlarLixi is higher than BIAsp 30, this is partially offset by the reduced dosing frequency with iGlarLixi.
These findings support recent cost-effectiveness assessments comparing iGlarLixi with other BI plus GLP-1 RA combinations for the treatment of T2D in post–BI and post–GLP-1 RA settings [22, 23]. However, these studies used estimated relative treatment effects from indirect treatment comparisons to inform the model; the present study uses direct observations from SoliMix, the first randomized trial comparing BI and the GLP-1 RA fixed-ratio combination with premix insulin in adults living with T2D advancing from BI plus one or two OADs (metformin with or without SGLT2 inhibitors). Because results from a clinical trial were used to inform the model, the impact of treatment burden (i.e., the number of daily injections people living with T2D must endure) on medication adherence was not considered. Adherence to anti-diabetic medication is associated with glycemic control [24], and increased regimen complexity has been associated with poorer adherence in people living with diabetes receiving anti-diabetic medications [9, 25]. Therefore, it seems plausible that the simpler once-daily iGlarLixi regimen would be associated with reduced treatment burden and better adherence than the twice-daily premix BIAsp 30 regimen, resulting in higher utility values and subsequent QALY estimates, but this remains to be confirmed. Additionally, the reduced nocturnal hypoglycemia and consequent improvement in quality of life observed with iGlarLixi versus BIAsp 30 observed in the SoliMix trial was not adequately captured by the present analysis and therefore not reflected in the QALYs gained.
Limitations
Because the analysis was conducted from a UK perspective, the results may not be applicable to other countries and currencies. Additionally, the data used in the analysis to predict long-term outcomes were relatively short term (26 weeks in the SoliMix trial); however, the robustness of the results were confirmed with sensitivity and scenario analyses. Owing to more rigorous monitoring and follow-up in the context of a randomized controlled trial, the incidence of severe hypoglycemia is generally lower than seen in routine clinical practice. As severe hypoglycemia rates were relatively low in the SoliMix trial, the impact on severe hypoglycemia in this analysis may be underestimated in those from an older—and therefore more frail—population and in those requiring third-party administration. In addition, this analysis did not consider the societal cost-effectiveness impact, including differences in administration burden with once-daily iGlarLixi versus twice-daily BIAsp 30 incurred by health care practitioners in community settings. Furthermore, the risk equations for progression of disease risk factors were based on the UKPDS 68 [13] and UKPDS 82 models [14]. The UKPDS risk equations are widely used in diabetes simulation models [26,27,28,29,30]. It should be noted that the UKPDS trial ended in 2007 and UKPDS equations may not fully reflect current clinical practice. Finally, these findings use clinical trial data of people receiving iGlarLixi and BIAsp 30 and do not evaluate how differences in adherence, timing of initiation and health care professional interaction may differ in routine clinical practice and any potential impact on outcomes observed. However, this limitation applies to all cost-effectiveness analyses applying trial data. Due consideration should also be given to other outcome measures, such as adverse events and patient-related outcome measures, when advancing diabetes therapy.
Conclusion
Over the lifetime of individuals living with T2D suboptimally controlled on BI therapy, iGlarLixi was associated with improved clinical outcomes at higher costs relative to premix BIAsp 30. At a WTP threshold of £20,000 per QALY gained, iGlarLixi was considered to be cost-effective versus premix BIAsp 30 from the perspective of the UK NHS.
References
Diabetes UK. Diabetes statistics. https://www.diabetes.org.uk/professionals/position-statements-reports/statistics. Accessed 28 Jun 2021.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020; 43:487–93.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018; 41:2669–701.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44:S111–24.
Polinski JM, Kim SC, Jiang D, et al. Geographic patterns in patient demographics and insulin use in 18 countries, a global perspective from the multinational observational study assessing insulin use: understanding the challenges associated with progression of therapy (MOSAIc). BMC Endocr Disord. 2015;15:46.
Jabbar A, Abdallah K, Hassoun A, et al. Patterns and trends in insulin initiation and intensification among patients with type 2 diabetes mellitus in the Middle East and North Africa region. Diabetes Res Clin Pract. 2019;149:18–26.
Meece J. Basal insulin intensification in patients with type 2 diabetes: a review. Diabetes Ther. 2018;9:877–90.
Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med. 2005;20:479–82.
Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682–9.
Chang P. Datamonitor Healthcare—diabetes type 2 disease analysis report. https://pharmastore.informa.com/product/disease-analysis-type-2-diabetes/. Accessed 21 Dec 2021.
Rosenstock J, Emral R, Sauque-Reyna L, et al. Advancing therapy in suboptimally controlled basal insulin–treated type 2 diabetes: clinical outcomes with iGlarLixi versus premix BIAsp 30 in the SoliMix randomized controlled trial. Diabetes Care. 2021;44:2361–70.
European Medicines Agency. Suliqua [summary of product characteristics]. https://www.ema.europa.eu/en/medicines/human/EPAR/suliqua. Accessed 8 Apr 2021.
Clarke PM, Gray AM, Briggs A, UK Prospective Diabetes Study (UKDPS) Group, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47:1747–59.
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–33.
Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20:S5-26.
Beaudet A, Clegg J, Thuresson P-O, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17:462–70.
National Institute for Healthcare and Excellence (NICE). Guide to the methods of technology appraisal 2013. Process and methods [PMG9]. https://www.nice.org.uk/process/pmg9/chapter/the-reference-case. Accessed 8 Apr 2021.
Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39:1972–80.
Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 trial. Diabetes Care. 2016;39:1318–28.
Ramos M, Cummings MH, Ustyugova A, Raza SI, de Silva SU, Lamotte M. Long-term cost-effectiveness analyses of empagliflozin versus oral semaglutide, in addition to metformin, for the treatment of type 2 diabetes in the UK. Diabetes Ther. 2020;11:2041–55.
McCrimmon RJ, Al Sifri S, Emral R, et al. Advancing therapy with iGlarLixi versus premix BIAsp 30 in basal insulin-treated type 2 diabetes: design and baseline characteristics of the SoliMix randomized controlled trial. Diabetes Obes Metab. 2021;23:1221–31.
McCrimmon RJ, Falla E, Sha JZ, et al. Cost-effectiveness of iGlarLixi in people with type 2 diabetes mellitus suboptimally controlled on basal insulin plus metformin in the UK. Diabetes Ther. 2021;12:3217–30.
McCrimmon RJ, Lamotte M, Ramos M, Alsaleh AJO, Souhami E, Lew E. Cost-effectiveness of iGlarLixi versus iDegLira in type 2 diabetes mellitus inadequately controlled by GLP-1 receptor agonists and oral antihyperglycemic therapy. Diabetes Ther. 2021;12:3231–41.
Doggrell SA, Warot S. The association between the measurement of adherence to anti-diabetes medicine and the HbA1c. Int J Clin Pharm. 2014;36:488–97.
Lokhandwala T, Smith N, Sternhufvud C, Sörstadius E, Lee WC, Mukherjee J. A retrospective study of persistence, adherence, and health economic outcomes of fixed-dose combination vs. loose-dose combination of oral anti-diabetes drugs. J Med Econ. 2016;19:203–12.
Gæde P, Johansen P, Tikkanen CK, Pollock RF, Hunt B, Malkin SJP. Management of patients with type 2 diabetes with once-weekly semaglutide versus dulaglutide, exenatide ER, liraglutide and lixisenatide: a cost-effectiveness analysis in the Danish setting. Diabetes Ther. 2019;10:1297–317.
Capehorn M, Hallén N, Baker-Knight J, Glah D, Hunt B. Evaluating the cost-effectiveness of once-weekly semaglutide 1 mg versus empagliflozin 25 mg for treatment of patients with type 2 diabetes in the UK setting. Diabetes Ther. 2021;12:537–55.
Ramos M, Foos V, Ustyugova A, Hau N, Gandhi P, Lamotte M. Cost-effectiveness analysis of empagliflozin in comparison to sitagliptin and saxagliptin based on cardiovascular outcome trials in patients with type 2 diabetes and established cardiovascular disease. Diabetes Ther. 2019;10:2153–67.
Ramos M, Cummings MH, Ustyugova A, Raza SI, de Silva SU, Lamotte M. Long-term cost-effectiveness analyses of empagliflozin versus oral semaglutide, in addition to metformin, for the treatment of type 2 diabetes in the UK. Diabetes Ther. 2020;11:2041–55.
Ehlers LH, Lamotte M, Monteiro S, et al. The cost-effectiveness of empagliflozin versus liraglutide treatment in people with type 2 diabetes and established cardiovascular disease. Diabetes Ther. 2021;12:1523–34.
Royal Pharmaceutical Society. About Medicines Complete. https://about.medicinescomplete.com/help/. Accessed 31 May 2021.
Acknowledgements
Funding
This study was sponsored by Sanofi, Paris, France. All publication costs, including the journal’s Rapid Service Fee, were funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors were responsible for the conception, study design, and acquisition, analysis, and interpretation of data. All authors contributed substantially to drafting and revising of the manuscript. All authors have reviewed and approved the final version of the manuscript for submission to Diabetes Therapy and agree to be accountable for its contents.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Martin Bell and Vanessa Gross of Curo (part of Envision Pharma Group) and was funded by Sanofi.
Disclosures
Rory J. McCrimmon is a member of the advisory panel for Novo Nordisk and Sanofi, a board member for NHS Tayside Health Board, and an employee at University of Dundee, and has received research support from Diabetes UK, EU Horizon 2020, JDRF, Medical Research Council/Wellcome Trust, and Novo Nordisk. Amar Puttanna has received honoraria, travel expenses, or conference registration fees from AstraZeneca, Eli Lilly, MSD, Napp, Novo Nordisk, Sanofi, and Takeda, and has participated in advisory boards for AstraZeneca, Eli Lilly, Janssen, Menarini, and Novo Nordisk. Karen Palmer, Abdul Jabbar Omar Alsaleh, Amar Puttanna and Elisheva Lew are employees of and stockholders in Sanofi.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
McCrimmon, R.J., Palmer, K., Alsaleh, A.J.O. et al. Cost-Effectiveness of iGlarLixi Versus Premix BIAsp 30 in Patients with Type 2 Diabetes Suboptimally Controlled by Basal Insulin in the UK. Diabetes Ther 13, 1203–1214 (2022). https://doi.org/10.1007/s13300-022-01267-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01267-3