Abstract
Introduction
A cost-effectiveness analysis was conducted comparing a fixed-ratio combination (FRC) of insulin glargine 100 units/mL plus lixisenatide (iGlarLixi) versus the FRC of insulin degludec plus liraglutide (iDegLira) and the free-combination comparators insulin glargine plus dulaglutide (iGlar plus Dula) and basal insulin plus liraglutide (BI plus Lira).
Methods
The IQVIA Core Diabetes Model was used to estimate lifetime costs and outcomes for a cohort of patients with type 2 diabetes mellitus (T2DM) from the UK healthcare perspective. Initial clinical data for iGlarLixi were based on the randomized, controlled LixiLan-L trial and the relative treatment effects for comparators were based on an indirect treatment comparison using data from the AWARD-9 (iGlar plus Dula), LIRA ADD2 BASAL (BI plus Lira), and DUAL V (iDegLira) trials. Costs were derived from publicly available sources. Lifetime costs (in British Pound Sterling [£]) and quality-adjusted life-years (QALYs) were predicted; net monetary benefit (NMB) for iGlarLixi versus comparators was derived using a willingness-to-pay threshold of £20,000. Extensive scenario and sensitivity analyses were conducted.
Results
Estimated costs were lowest with iGlarLixi (£31,295) compared with iGlar plus Dula (£38,790), iDegLira (£40,179), and BI plus Lira (£42,467). Total QALYs gained were identical with iGlarLixi and iDegLira (8.438), and comparable with iGlar plus Dula (8.439) and BI plus Lira (8.466). NMB for iGlarLixi was positive versus all comparators (£10,603.86 vs. BI plus Lira; £7,466.24 vs. iGlar plus Dula; £8.874.11 vs. iDegLira).
Conclusion
In patients with T2DM with suboptimal glycemic control on basal insulin, iGlarLixi provides very similar outcomes and substantial cost savings, compared with other fixed and free combinations of insulins plus glucagon-like peptide-1 receptor agonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with type 2 diabetes mellitus (T2DM) who do not achieve target levels of glycated hemoglobin (HbA1c) despite escalating therapy and treatment with basal insulin (BI) analogs require further intensification of the insulin regimen to achieve glycemic control. |
The combination of injectable glucagon-like peptide-1 receptor agonists (GLP-1 RA) and BI therapy has been shown to improve glycemic control compared with insulin alone, but separate injectables are associated with increased regimen complexity, additional costs, and poorer adherence. |
As no economic evaluation of the available fixed-ratio combination (FRC) and free-combination regimens of BI plus GLP-1 RA has been undertaken to date, a comparative cost-effectiveness comparison of the FRCs insulin analog glargine plus lixisenatide (iGlarLixi) and insulin degludec plus liraglutide (iDegLira) with the free-combination comparators insulin glargine plus dulaglutide (iGlar plus Dula) and basal insulin plus liraglutide (BI plus Lira) was conducted. |
What was learned from this study? |
iGlarLixi was less costly (iGlarLixi: £31,295, iGlar plus Dula: £38,790, iDegLira: £40,179, BI plus Lira: £42,467) and total quality-adjusted life-years gained were identical with iGlarLixi and iDegLira (8.438), and comparable with iGlar plus Dula (8.439) and BI plus Lira (8.466). |
In patients with T2DM with suboptimal glycemic control on basal insulin, iGlarLixi provides comparable outcomes and substantial cost savings, compared with other fixed and free combinations of BI plus GLP-1 RA, and was considered to be cost-effective over a lifetime time horizon. |
Introduction
Diabetes remains one of the most impactful global health threats, and clinical management of people with type 2 diabetes mellitus (T2DM) represents a major public health challenge and a pressing economic burden for payers in the UK. Subsequent to lifestyle interventions and iterative dose escalation with oral antidiabetic drugs (OADs), some patients with T2DM do not achieve their individually recommended target levels of glycated hemoglobin (HbA1c) and may further escalate therapy and receive basal insulin (BI) analogs, in line with guidance from the American Diabetes Association, European Association for the Study of Diabetes, and National Institute for Health and Care Excellence (NICE) [1, 2]. However, despite the proven efficacy of BI regimens in clinical studies [3], a substantial proportion of patients with T2DM continue to experience suboptimal glycemic control with BI analog monotherapy, and further intensification of the insulin regimen is often required to achieve glycemic control [4, 5].
The development of injectable glucagon-like peptide-1 receptor agonists (GLP-1 RAs) offers a complementary mechanism of action to BI analogs through their action to reduce post-prandial glucose excursions by enhancing glucose-stimulated insulin secretion and delaying gastric emptying. In addition, GLP-1 RAs reduce appetite [6], thereby improving glycemic control while minimizing the side effects of hypoglycemia and weight gain. The combination of GLP-1 RA and BI therapy has been shown to improve glycemic control compared with insulin alone [7–9]. However, combining both of these agents as separate injectables is associated with increased regimen complexity and additional costs [10], and may be associated with poorer adherence [11]. The combination of BI with GLP-1 RAs administered as a single fixed-ratio combination (FRC) injection offers additional convenience, which facilitates dual treatment initiation, simplification of the dosing schedule, and easy titration for physicians and patients [12, 13].
Two FRCs of insulin plus GLP-1 RA are currently approved for use in the EU. The FRC iDegLira, comprising insulin degludec and the GLP-1 RA liraglutide, is approved in the EU for use in adults with insufficiently controlled T2DM as an adjunct to diet and exercise, in addition to other OADs [14]. In the DUAL V clinical trial, which assessed patients with T2DM with inadequate glycemic control during therapy with insulin glargine plus metformin, those who received iDegLira had a significantly improved HbA1c reduction, hypoglycemic event rate, and body weight outcomes compared with insulin glargine [15]. The FRC iGlarLixi (Suliqua®; Sanofi, Paris, France) comprises the long-acting insulin analog glargine 100 units/mL and the GLP-1 RA lixisenatide, and is approved in the EU for use in adults with insufficiently controlled T2DM as an adjunct to diet and exercise, in addition to metformin, with or without sodium-glucose co-transporter-2 inhibitors [16]. The LixiLan-L clinical trial (ClinicalTrials.gov: NCT02058160) demonstrated that in patients with T2DM who had suboptimal glycemic control despite BI therapy (with or without metformin), iGlarLixi was associated with a significant reduction in HbA1c and significantly more patients achieving target HbA1c < 7.0% compared with insulin glargine alone (55 vs. 30% of patients; P < 0.0001), and was not associated with an increased risk of hypoglycemia [17].
A comprehensive economic analysis comparing available BI plus GLP-1 RA FRCs and free-combination regimens of BI plus GLP-1 RA in patients with T2DM suboptimally controlled with BI has not previously been conducted. We therefore conducted a cost-effectiveness comparison of the FRCs iGlarLixi and iDegLira with the free-combination comparators insulin glargine plus dulaglutide 1.5 mg (iGlar plus Dula) and basal insulin plus liraglutide 1.8 mg (BI plus Lira).
Methods
Study Overview
The IQVIA Core Diabetes Model (CDM) is a non-product-specific cohort simulation model that determines long-term health outcomes and cost consequences of interventions in type 1 and type 2 diabetes mellitus [18, 19]. The CDM is designed to take surrogate endpoints (HbA1c, blood pressure, lipids, weight, hypoglycemia) and translate them into long-term health economic outcomes (life expectancy, micro- and macrovascular complications, quality-adjusted life expectancy, total costs). The clinical setting for this model was patients with T2DM with suboptimal glycemic control on a BI analog (with or without ≥ 2 OADs) who were considered appropriate for treatment intensification with a GLP-1 RA. The baseline patient characteristics were sourced from the LixiLan-L trial [17]. Outcomes over a lifetime time horizon were estimated using version 9.5 of the IQVIA CDM; model structure and analysis is described in further detail in the complementary article also published in this issue [20].
This cost-effectiveness analysis was conducted in line with NICE guidance and from the perspective of the UK National Health Service, using a hypothetical cohort of 1000 patients, a lifetime time horizon, and an annual discount rate of 3.5% for both costs and outcomes [21].
Model Inputs and Structure
Baseline characteristics were based primarily on those reported in the LixiLan-L clinical trial [17]; baseline HbA1c was 8.08%. For laboratory parameters, waist circumference, waist-to-hip ratio, and proportion of patients with severe vision loss, default values in the CDM were used (Table 1). The treatment effect of iGlarLixi on HbA1c in the first year of the model was based on the outcomes observed in the LixiLan-L trial [17] (Table 2); an indirect treatment comparison (ITC) using Bucher methodology was conducted to provide an estimate of relative treatment effects on HbA1c for iGlar plus Dula, BI plus Lira, and iDegLira [22]. Data from the following phase 3 trials in patients with suboptimal glycemic control with BI were used to conduct the ITC: the AWARD-9 study (NCT02152371), assessing iGlar plus Dula [23]; the LIRA ADD2 BASAL study (NCT02964247), assessing BI plus Lira [24]; and the DUAL V study (NCT01952145), assessing iDegLira [15] (Table 2). After the first year of treatment, the progression of HbA1c was predicted based on the UK Prospective Diabetes Study (UKPDS) 68 risk equation [25]; for cardiovascular diseases and mortality, the UKPDS 82 risk equations were used [26]. As the effect on body mass index (BMI) was not reported in primary publications for relevant trials, it was assumed that there was no difference to the effect on BMI between interventions in the base case; this assumption was tested in sensitivity analyses. All therapies were assumed to be followed by a rescue therapy (addition of a rapid-acting insulin) after patients exceeded a threshold of HbA1c 8.08% (i.e. their HbA1c has returned to baseline levels). The estimated reduction in HbA1c following the addition of rescue therapy was approximated based on the GetGoal Duo-2 trial [27], in which the addition of bolus insulin to BI reduced HbA1c by 0.6%. Formal comparisons of hypoglycemia were limited by between-study differences in the definitions of hypoglycemia.
For the derivation of quality-adjusted life-years (QALYs), an additive “core default method” approach was used: for patients with multiple comorbidities (e.g. history of stroke and myocardial infarction [MI]), the lowest utility value was assigned, and event disutilities were then added for events that occur in that year, resulting in an annual utility score for each simulated patient [19]. Utility and disutility weightings were taken from previously published cost-effectiveness evaluations using the CDM [28] (see Electronic Supplementary Material [ESM] Table S1).
Cost Data
Direct medical costs, comprising drug costs, administration costs, glucose monitoring costs, and costs of T2DM complications (cardiovascular, renal, acute events, eye disease, neuropathy, foot ulcer, and amputation), were calculated for each year of therapy, based on published literature and UK national sources (Table 3). Treatment costs for insulins were calculated based on the daily doses reported by source trials. The cost of insulin glargine was conservatively assumed to equal the cost of the biosimilar insulin glargine, Semglee® (Biocon Biologics Ltd., Bengaluru, India). In the EU, iGlarLixi is available as two FRCs: 100 units/mL insulin glargine plus 50 µ/mL lixisenatide (Suliqua® SoloStar pen 10–40 units, hereinafter “iGlarLixi 100/50”) and 100 units/mL insulin glargine plus 33 µ/mL lixisenatide (Suliqua® SoloStar pen 30–60 units, hereinafter “iGlarLixi 100/33”). The cost of iGlarLixi in the first year was estimated based on assumed use of the iGlarLixi 100/50 FRC for 3 months followed by use of iGlarLixi 100/33 FRC for the remaining 9 months of that first year. From the second year onward, it was assumed that only the iGlarLixi 100/33 formulation was used. This is a conservative approach considering the titration period and final dose of 46.7 IU reached at the end of the treatment period in the LixiLan-L trial.
All patients were assumed to also be receiving concurrent metformin as oral diabetes therapy. Following treatment switch to rescue therapy (addition of a rapid-acting insulin, after patients’ HbA1c returned to baseline levels of 8.08%), costs resulting from the addition of rapid-acting insulin were added. All costs were reported in 2020 British Pound Sterling (GBP [£]) and, if necessary, inflated to 2020 costs using the Hospital and Community Health Service Index from the Personal Social Services Research Unit [29]. Latest official tariffs were used where applicable.
Analyses
Incremental differences in costs and QALYs were obtained for iGlarLixi versus BI plus Lira, iGlar plus Dula, and iDegLira; incremental cost-effectiveness ratio (ICER) estimates were calculated for iGlarLixi relative to each comparator. Additionally, cost impact was assessed using the net monetary benefit (NMB) approach, conservatively based on the lower range (£20,000) of the commonly accepted UK willingness-to-pay (WTP) threshold of £20,000–30,000 [21]. Positive NMB values indicate that an intervention is cost-effective compared with the alternative at a given WTP threshold [30].
Scenario analyses were performed on key parameters to assess the robustness of the base case findings (ESM Table S2). A probabilistic sensitivity analysis (PSA), including 1000 model iterations in which key parameter inputs were altered within plausible distribution estimates, was also conducted to capture uncertainty and assess the imprecision of the results. Probabilistic distribution of key transition probabilities (for MI, stroke, congestive heart failure, and angina) were applied by bootstrap sampling around the 95% confidence interval of the regression coefficient. For utilities and treatment effects, mean and standard error values were used to generate random sampling within a beta-distribution function. Direct costs (excluding acquisition costs, which were assumed to be fixed) were randomly sampled based on log-normal distribution within a 10% variance.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Base Case Analysis
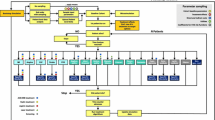
Total QALYs gained were identical with iGlarLixi and iDegLira (8.438), and almost identical with iGlar plus Dula (8.439) and BI plus Lira (8.466) (Table 4). Estimated total costs were lowest with iGlarLixi (£31,295), compared with iGlar plus Dula (£38,790), iDegLira (£40,179), and BI plus Lira (£42,467). Treatment switching to rescue therapy occurred after year 5 with iGlarLixi, iGlar plus Dula, and iDegLira, and after year 6 with BI plus Lira; predicted HbA1c levels with each comparator converged over time. Estimated event rates for key diabetes-related complications were comparable between comparators (ESM Fig. S1). Owing to difficulties when interpreting ICERs in the south-west quadrant (Fig. 1), NMB was estimated at a WTP threshold of £20,000. For all comparisons, the NMB of iGlarLixi was positive; the NMB of iGlarLixi was £10,603.86 versus BI plus Lira, £7,466.24 versus iGlar plus Dula, and £8874.11 versus iDegLira (Table 4).
Base case cost-effectiveness planes and cost-effectiveness acceptability curves for iGlarLixi versus: a, b BI plus Lira, c, d iGlar plus Dula, e, f iDegLira. BI basal insulin, Dula dulaglutide, ICER incremental cost-effectiveness ratio, iDegLira insulin degludec plus liraglutide, iGlar insulin glargine, iGlarLixi insulin glargine 100 units/mL plus lixisenatide, Lira liraglutide, PSA probabilistic sensitivity analysis, QALY quality-adjusted life-years, WTP willingness to pay
The key driver for cost savings with iGlarLixi was the estimated annual acquisition costs (£1011 in the first year, £921 in subsequent years) compared with BI plus Lira (£1774 annually), iGlar plus Dula (£1488 annually), and iDegLira (£1590 annually). Costs associated with management and complications were similar between comparators.
Scenario Analyses
Multiple scenario and one-way analyses were conducted to assess the robustness of the base case model assumptions (ESM Table S3). Base case results remained robust to parameter and assumption variation in all scenario analyses. The NMB for iGlarLixi ranged from £2818 to £16,163 versus BI plus Lira; from £2296 to £11,311 versus iGlar plus Dula; and from £2775 to £13,470 versus iDegLira.
Probabilistic Sensitivity Analysis
For iGlarLixi versus each comparator, all iterations of the PSA demonstrated cost savings for iGlarLixi, but a substantial proportion of iterations resulted in fewer QALYs gained for iGlarLixi (ranging from 52% of iterations vs. iDegLira, to 60% of iterations vs. BI plus Lira; Fig. 1). Nevertheless, at a WTP threshold of £20,000/QALY, iGlarLixi was cost-effective in approximately 98% of iterations in each comparison (Fig. 1b).
Discussion
This study evaluated the cost-effectiveness of iGlarLixi and demonstrated that iGlarLixi provides comparable efficacy with iDegLira, BI plus Lira, and iGlar plus Dula, but is less costly than these comparators, and is therefore likely to represent a cost-effective alternative for healthcare payers. Extensive scenario analyses and PSA consistently supported the base case findings, demonstrating the robustness of these outcomes.
Previous publications have reported on cost-effectiveness estimates for iDegLira versus iGlarLixi from Italian and Czech Republic perspectives. In the Italian estimate, iDegLira was associated with an incremental cost of €930 and a gain of 0.13 QALYs [31], while in the Czech Republic the incremental cost was estimated to be CZK 94,029 (£3164) for the iGlarLixi 100/33 (30–60) pen and CZK 47,058 (£1583) for the iGlarLixi 100/50 (10–40) pen, with a gain of 0.14 QALYs [32]. These cost estimates are qualitatively different from those presented in this UK analysis, which consistently demonstrated iGlarLixi to be less expensive than comparators, but in both analyses, the ICER estimates for iDegLira were well below local WTP thresholds. However, both analyses relied on the same indirect comparison, taking the treatment effect from the DUAL II trial for iDegLira; patient characteristics in DUAL V are closer to those in LixiLan-L [33]. Furthermore, these analyses assumed that the treatment effect in HbA1c and BMI was maintained over 5 years, while in our analysis we assumed that after the first year, the progression of HbA1c was predicted with the UKPDS 68 equation. Upon treatment intensification after 5 years, HbA1c 7% was assumed for the remainder of patients’ lifetimes. Our analysis assumed patients were switched to rescue therapy once they reached their HbA1c baseline value again, and HbA1c reduction of 0.6% was applied to rescue therapy for the first year with the progression of HbA1c predicted from UKPDS 68 for the remainder of patients’ lifetimes. For utility values, we applied the lowest-state utility associated with existing comorbidities.
QALY outcomes were very similar with each comparator in this analysis. NMB can more accurately capture cost benefits when the ICER lies in the south-west quadrant of the effectiveness plane and incremental differences are marginal. NMB is being used increasingly in health economic evaluation; indeed, it was recently used by NICE to rank BI therapies in the economic report underpinning their guidance for diagnosis and management of type 1 diabetes [34]. In our analysis, the base case and all scenario analyses had positive NMB estimates, indicating that the cost savings with iGlarLixi outweighed the value of the marginal (and likely clinically non-meaningful) differences in QALYs.
There are several limitations with this approach. The model relies on short-term (26–30 weeks) data from two trials to extrapolate long-term projections; however, only long-term analyses are suitable to capture the full economic and clinical impact of chronic conditions, as recommended by the American Diabetes Association [35]. Similarly, the model outcomes assume consistent and reliable treatment effects over time; such observations have been difficult to obtain in real-world clinical practice owing to issues with adherence and persistence to therapy [36–38], but this limitation is likely to apply equally to all comparators. A further limitation for this analysis is the lack of direct head-to-head trial data to inform the model. In the absence of such data, relative efficacy values for comparators were derived from an ITC. The base case considered only the relative HbA1c treatment effect while the BMI effect was assumed to be equal between comparators, and hypoglycemia rates were predicted with risk equations owing to inconsistent definitions used in studies. As BMI and hypoglycemia are core components of the progression of diabetes complications over time, and are an important measure of the impact of treatment on patients, these parameters were tested in sensitivity analyses. The overall conclusion remained unchanged. Additionally, the GetGoal Duo-2 trial served as proxy to estimate the efficacy of the rescue therapy. It is likely that this assumption underestimated treatment efficacy. It should further be noted that this assessment was based on the current costs of the therapies and would therefore be subject to change if the pricing structure for the investigated therapies changes.
Conclusion
In conclusion, with a positive NMB in an increasingly economically constrained environment, these analyses show that iGlarLixi represents good value for money to payers while remaining a relevant therapeutic choice for clinicians and their patients.
References
Blonde L, Meneghini L, Peng XV, et al. Probability of achieving glycemic control with basal insulin in patients with type 2 diabetes in real-world practice in the USA. Diabetes Ther. 2018;9(3):1347–58.
Peng XV, McCrimmon RJ, Shepherd L, et al. Glycemic control following GLP-1 RA or basal insulin initiation in real-world practice: a retrospective, observational, longitudinal cohort study. Diabetes Ther. 2020;11(11):2629–45.
Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371(9626):1753–60.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–93.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701.
Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–42.
Aroda VR. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20(Suppl 1):22–33.
Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care. 2013;36(9):2489–96.
Maiorino MI, Chiodini P, Bellastella G, Capuano A, Esposito K, Giugliano D. Insulin and glucagon-like peptide 1 receptor agonist combination therapy in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2017;40(4):614–24.
Tran E. Fixed-ratio combinations. Clin Diabetes. 2017;35(4):242–6.
Lokhandwala T, Smith N, Sternhufvud C, Sorstadius E, Lee WC, Mukherjee J. A retrospective study of persistence, adherence, and health economic outcomes of fixed-dose combination vs. loose-dose combination of oral anti-diabetes drugs. J Med Econ. 2016;19(3):203–12.
Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and Lixisenatide Monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39(11):2026–35.
Gough SC, Bode BW, Woo VC, et al. One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension to a 26-week main trial. Diabetes Obes Metab. 2015;17(10):965–73.
European Medicines Agency. Xultophy summary of product characteristics. 2014. https://www.ema.europa.eu/en/documents/product-information/xultophy-epar-product-information_en.pdf. Accessed 8 Apr 2021.
Lingvay I, Perez Manghi F, Garcia-Hernandez P, et al. Effect of insulin glargine up-titration vs insulin Degludec/Liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315(9):898–907.
European Medicines Agency. Suliqua summary of product characteristics. 2017. https://www.ema.europa.eu/en/documents/product-information/suliqua-epar-product-information_en.pdf. Accessed 8 Apr 2021.
Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus Lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39(11):1972–80.
Palmer AJ, Roze S, Valentine WJ, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5-26.
McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE diabetes model. Value Health. 2014;17(6):714–24.
McCrimmon, Lamotte, Ramos et al. Cost-Effectiveness of iGlarLixi Versus iDegLira in Type 2 Diabetes Mellitus Inadequately Controlled by GLP-1 Receptor Agonists and Oral Antihyperglycemic Therapy. Diabet Ther. 2021, In Press.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013—Process and methods [PMG9]. 2013. https://www.nice.org.uk/process/pmg9/chapter/the-reference-case. Accessed 8 Apr 2021.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91.
Pozzilli P, Norwood P, Jodar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab. 2017;19(7):1024–31.
Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056–64.
Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–59.
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33.
Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing Lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 trial. Diabetes Care. 2016;39(8):1318–28.
Ramos M, Cummings MH, Ustyugova A, Raza SI, de Silva SU, Lamotte M. Long-term cost-effectiveness analyses of empagliflozin versus oral semaglutide, in addition to metformin, for the treatment of type 2 diabetes in the UK. Diabetes Ther. 2020;11(9):2041–55.
Curtis LA, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: University of Kent Personal Social Services Research Unit (PSSRU). https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2020/. Accessed 8 Apr 2021.
York Health Economics Consortium. Net Monetary Benefit. 2016. https://yhec.co.uk/glossary/net-monetary-benefit/. Accessed 6 July 2021.
Pohlmann J, Montagnoli R, Lastoria G, Parekh W, Markert M, Hunt B. Value for money in the treatment of patients with type 2 diabetes mellitus: assessing the long-term cost-effectiveness of IDegLira versus iGlarLixi in Italy. Clinicoecon Outcomes Res. 2019;11:605–14.
Pohlmann J, Russel-Szymczyk M, Holik P, Rychna K, Hunt B. Treating patients with type 2 diabetes mellitus uncontrolled on basal insulin in the Czech Republic: cost-effectiveness of IDegLira versus iGlarLixi. Diabetes Ther. 2019;10(2):493–508.
Evans M, Billings LK, Hakan-Bloch J, et al. An indirect treatment comparison of the efficacy of insulin degludec/liraglutide (IDegLira) and insulin glargine/lixisenatide (iGlarLixi) in patients with type 2 diabetes uncontrolled on basal insulin. J Med Econ. 2018;21(4):340–7.
National Institute for Healthcare and Excellence. Type 1 diabetes in adults: diagnosis and management—Insulin therapy. NICE guideline NG17—Economic model report. Draft for consulation. 2021. https://www.nice.org.uk/guidance/gid-ng10159/documents/economic-report. Accessed 8 Apr 2021.
American Diabetes Association. Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–5.
Tofe S, Arguelles I, Mena E, et al. Real-world GLP-1 RA therapy in type 2 diabetes: a long-term effectiveness observational study. Endocrinol Diabetes Metab. 2019;2(1):e00051.
Guerci B, Charbonnel B, Gourdy P, et al. Efficacy and adherence of glucagon-like peptide-1 receptor agonist treatment in patients with type 2 diabetes mellitus in real-life settings. Diabetes Metab. 2019;45(6):528–35.
Lin J, Lingohr-Smith M, Fan T. Real-world medication persistence and outcomes associated with basal insulin and glucagon-like peptide 1 receptor agonist free-dose combination therapy in patients with type 2 diabetes in the US. Clinicoecon Outcomes Res. 2017;9:19–29.
NHS Scotland. Scottish Diabetes Survey NHS Scotland. 2017. https://www.diabetesinscotland.org.uk/wp-content/uploads/2020/02/Diabetes-in-Scotland-website-Scottish-Diabetes-Survey-2017.pdf. Accessed 30 July 2021.
NHS Scotland. Monitoring and evaluating Scotland’s alcohol strategy: Monitoring Report 2017. NHS Health Scotland. 2017. http://www.healthscotland.scot/media/1449/mesas-final-report_english1.pdf. Accessed 30 July 2021.
Rubino A, Rousculp MD, Davis K, Wang J, Girach A. Diagnosed diabetic retinopathy in France, Italy, Spain, and the United Kingdom. Prim Care Diabetes. 2007;1(2):75–80.
British Medical Association and the Royal Pharmaceutical Society. British National Formulary. 2020. https://about.medicinescomplete.com/help/. Accessed 31 May 2021.
Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–70.
Lauridsen JT, Lonborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645–50.
Foos V, McEwan P. Conversion of hypoglycemia utility decrements from categorical units reflecting event history into event specific disutility scores applicable to diabetes decision models. Value Health. 2018;21:S223.
Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14(3):217–30.
Acknowledgements
Funding
This study was sponsored by Sanofi. All publication costs, including the journal’s Rapid Service fee, were funded by Sanofi.
Medical Writing and Editorial Assistance
Medical writing support was provided by Martin Bell and Vanessa Gross of Curo (part of Envision Pharma Group) and was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors were responsible for the conception, study design, and to the acquisition, analysis and interpretation of data. All authors contributed substantially to drafting and revising of the manuscript. All authors have reviewed and approved the final version of the manuscript for submission to Diabetes Therapy, and agree to be accountable for its contents.
Disclosures
Rory J. McCrimmon is a member of the advisory panel for Sanofi and Novo Nordisk, a board member for NHS Tayside Health Board, an employee at University of Dundee, and has received research support from the Medical Research Council, Wellcome, EU Horizon 2020, JDRF, Diabetes UK, and Novo Nordisk. Edel Falla and Jo Zhou Sha are employees of IQVIA, which received consulting fees for adapting the IQVIA Core Diabetes Model to allow the analyses reported in this manuscript. Abdul Jabbar Omar Alsaleh has received independent contractor funding from Sanofi for this analysis. Elisheva Lew, Richard Hudson, and Karen Palmer are employees and stockholders of Sanofi. Mike Baxter was an employee and stockholder of Sanofi at the time of the study.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
McCrimmon, R.J., Falla, E., Sha, J.Z. et al. Cost-Effectiveness of iGlarLixi in People with Type 2 Diabetes Mellitus Suboptimally Controlled on Basal Insulin Plus Metformin in the UK. Diabetes Ther 12, 3217–3230 (2021). https://doi.org/10.1007/s13300-021-01159-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01159-y