Abstract
Introduction
Gender differences in risk factors and treatment outcomes for type 2 diabetes mellitus (T2DM) may exist. We used the REALI European database to investigate whether there were gender-specific differences in baseline characteristics and clinical outcomes among patients with inadequately controlled T2DM initiated on insulin glargine 300 U/ml (Gla-300).
Methods
Data were pooled from 14 multicentre, prospective, interventional and non-interventional studies. Impact of gender on glycaemic control, insulin dose, body weight and hypoglycaemia was evaluated after 12 and 24 weeks of Gla-300 treatment.
Results
Women (N = 3857) were older than men (N = 4376) (median age, 65.0 versus 63.0 years), with greater mean body mass index (32.5 versus 31.6 kg/m2) and lower median estimated glomerular filtration rate (77.5 versus 84.0 ml/min/1.73 m2). Peripheral arterial disease and a history of myocardial infarction were more frequent in men (20.1% versus 11.7% and 12.0% versus 5.8%, respectively). At baseline, mean haemoglobin A1c (HbA1c) was 8.74% in men and 8.79% in women. Least square (LS) mean (95% CI) reduction in HbA1c from baseline to week 24 was − 1.17% (− 1.21 to − 1.13) in men and − 1.07% (− 1.11 to − 1.02) in women, resulting in a LS mean difference of − 0.10% (− 0.15 to − 0.05; p < 0.0001). At 24 weeks, 21.6% of women and 27.2% of men achieved target HbA1c of < 7.0% (p < 0.001; chi-square). Reported incidence for symptomatic (8.5% versus 8.7%) and severe (0.3% versus 0.5%) any-time-of-the-day or symptomatic (2.4% versus 1.8%) and severe (0.1% versus 0.2%) nocturnal hypoglycaemia was overall low and comparable between men and women. Changes in daily Gla-300 dose and body weight were also similar.
Conclusion
Despite some gender differences in baseline characteristics, Gla-300 treatment improved glycaemic control, with overall low hypoglycaemia incidences in both men and women. However, women had statistically significantly lower HbA1c reductions than men, although these differences were clinically modest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Increasing evidence suggests that gender affects the pathophysiology, epidemiology, symptoms, course and response to therapy in type 2 diabetes mellitus (T2DM). |
Insulin glargine 300 U/ml (Gla-300) is a second-generation, long-acting basal insulin analogue that has been extensively evaluated in several large, multicentre, randomised controlled trials; there is however a dearth of data describing the impact of gender on the effectiveness and safety of Gla-300, particularly in settings close to clinical practice. |
What was learned from the study? |
In the REALI pooled analysis of 14 interventional and non-interventional studies conducted among European patients with uncontrolled T2DM, both men and women achieved clinically important improvements in glycaemic control after Gla-300 treatment initiation, without notable gender differences in reported hypoglycaemia event rates and incidence, body weight and insulin dose changes. |
Our findings support the use of Gla-300 in both women and men with inadequately controlled T2DM; however, to optimise diabetic management, an individualised treatment approach taking gender into account may still be considered. |
Introduction
Increasing evidence suggests that gender affects the pathophysiology, epidemiology, symptoms, course and response to therapy in type 2 diabetes mellitus (T2DM) [1,2,3,4,5]. For instance, women with T2DM generally have poorer glycaemic control and are less likely to reach the goals for haemoglobin A1c (HbA1c) compared with men [1, 2, 6]. In addition, more women than men die of diabetes on a global scale: 2.3 versus 1.9 million in 2019 [7]. The relative risk of diabetes-related vascular complications is also substantially higher in women than men [8].
The mechanisms underlying gender-related differences of glycaemic control have several determinants, including gender-based differences in body fat distribution and hormones as well as slower glucose absorption in women [1, 2, 8, 9]. Varying disease outcomes may also be due to differences in treatment response and psychological factors as well as health care disparities between the genders [6, 10]. Not only are women with T2DM more likely to experience side effects from glucose-lowering agents such as hypoglycaemia, but some data indicated they were also less likely to be adherent to treatment than men [1, 10, 11]. In addition, women with T2DM appear to fare worse psychologically and suffer more from depression, anxiety and low energy levels compared with men, potentially contributing to the lower degree of achievement of HbA1c targets [10, 12]. Women have also experienced a poorer quality of diabetes care than men, with a lower likelihood to be monitored for diabetes complications [13]. Despite the potential role of gender in glycaemic control, there are currently no specific treatment guidelines differentiating between genders [6, 10].
Insulin glargine 300 U/ml (Gla-300) is a new generation, long-acting basal insulin analogue providing similar glycaemic control to that achieved with insulin glargine 100 U/ml (Gla-100), with a lower risk of hypoglycaemia and less weight gain [14]. Although the efficacy and safety of Gla-300 in people living with diabetes have been evaluated in several large, multicentre, randomised controlled trials (RCTs) [14], there is currently a dearth of data describing the impact of gender on the effectiveness and safety of Gla-300, particularly in settings close to clinical practice. Accordingly, we used the REALI European database to evaluate the association of gender with response to Gla-300 therapy, based on data from 14 non-interventional and interventional studies conducted in patients with T2DM uncontrolled on previous glucose-lowering therapy who initiated or switched to Gla-300.
Methods
Study Designs and Patient Populations
This analysis included pooled data from 14 multicentre, prospective, open-label studies [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] of a minimum duration of 24 weeks conducted among European adult patients with inadequately controlled T2DM who initiated Gla-300 treatment (Table 1). The rationale, methodology and a detailed description of the variables have been already provided in the published protocol of the REALI project [30].
For most included studies in the REALI pooled analysis, poor glycaemic control was defined as an HbA1c between 7.5 and 10.0%. In each study, Gla-300 was injected subcutaneously once daily, using a pre-filled insulin pen at the same time of the day ± 3 h if needed, as specified in the summary of product characteristics [31]. Two of the included studies (Take Control [22] and ITAS [23, 29]) were interventional, single-drug, two-arm studies, in which patients were randomised (1:1) according to Gla-300 titration to either a self- or physician-managed titration algorithm of Gla-300, whereas the others were non-interventional, single-arm studies, in which Gla-300 was initiated and titrated according to real-world clinical practice. All studies were performed in the ambulatory care setting, except COBALTA [24], which included a Gla-300 initiation at the hospital followed by an ambulatory use after discharge.
Patients included within the REALI analysis were either insulin-naïve or previously treated with insulin (basal insulin ± prandial insulin) with or without non-insulin glucose-lowering agents. There were no upper age limit restrictions in any trial. Common exclusion criteria included a diagnosis of type 1 diabetes, pregnancy and/or breastfeeding, a history of alcohol or drug abuse, the presence of any clinically relevant somatic or mental disease, stage 5 chronic kidney disease, known hypersensitivity or intolerance to Gla-300 or any of its excipients, and inability to self-measure blood glucose levels.
Protocols for all included studies were approved by the appropriate ethics committees, and the studies were conducted according to Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent. In this analysis, patient-level raw data were pooled from the multiple studies. The raw, de-identified datasets obtained were standardised for consistency in coding prior to pooling.
Outcomes
Efficacy outcomes evaluated in the pooled analysis were the changes in HbA1c and fasting plasma glucose (FPG) from baseline to week 12 and week 24 of Gla-300 treatment as well as the proportion of patients achieving HbA1c targets of < 7.0% (53.0 mmol/mol), < 7.5% (58.5 mmol/mol) and < 8.0% (63.9 mmol/mol) at week 24.
Safety endpoints included percentages of patients having at least one hypoglycaemic event at any time of day or during the night and hypoglycaemia event rates (events per patient-year). Nocturnal hypoglycaemia was evaluated to exclude potential confounders relating to daytime activities and meal intake. The definitions of hypoglycaemia were predetermined in the present pooled analysis. Severe hypoglycaemia was defined as any event requiring assistance from another person to actively administer carbohydrates or glucagon or to take other corrective actions [32]. Symptomatic hypoglycaemia was defined as an event during which typical symptoms of hypoglycaemia occurred (e.g., sweating, hunger, shakiness, palpitations).
The pooled analysis also evaluated changes in body weight and in the daily dose of Gla-300 from baseline to week 12 and week 24 of Gla-300 treatment.
Statistical Analyses
All outcome measures were analysed according to gender. The changes in HbA1c and FPG from baseline to weeks 12 and 24 of Gla-300 treatment were analysed using a mixed model for repeated measures, with fixed categorical effects of visit and gender as well as continuous fixed covariates of baseline HbA1c or FPG, baseline HbA1c or FPG value-by-visit interaction and gender-by-visit interaction. For these two efficacy endpoints, the least square (LS) mean differences between men and women and two-sided 95% confidence intervals (CIs) were estimated.
All other efficacy and safety endpoints as well as baseline demographic and disease characteristics were assessed descriptively, with categorical variables presented as counts and percentages, and continuous variables as mean, standard deviation (SD), median, and first and third quartiles.
Efficacy and safety assessments were based on all included patients who received at least one Gla-300 dose. There were missing patient baseline characteristics and missing outcome data in some studies; no imputation of missing data was performed. All statistical tests were two-sided, with a p value < 0.05 considered statistically significant. All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline Characteristics
A total of 8233 patients from 20 European countries comprised the pooled study population (4376 men [53.2%] and 3857 women [46.8%]), with a male:female ratio of 1.1. The gender ratio largely varied across the included studies, with the male:female ratio ranging from 0.8 to 2.1 (Table 1). Of the 8233 patients, 8046 (97.7%) received at least one dose of Gla-300 and were consequently included in the efficacy and safety analyses.
Patients’ baseline demographic and disease characteristics are summarised in Table 2. Compared to men, women had a higher median age as well as a higher mean body mass index at baseline. In addition, median levels of estimated glomerular filtration rate were lower in women compared to men. The study population was characterised by a history of long-standing T2DM, with more than half of women (54.7%) and men (52.1%) having a diabetes duration ≥ 10 years. Gla-100 was the most commonly used prior basal insulin across both gender subgroups, with a mean daily basal insulin dose higher in men. More men (72.6%) than women (66.6%) were treated with non-insulin glucose-lowering agents at baseline (most commonly with metformin), and this type of treatment did not notably change during the 24-week Gla-300 treatment period.
Peripheral arterial disease (PAD) and a history of myocardial infarction (MI) were more frequent in men than in women (20.1% versus 11.7% and 12.0% versus 5.8%, respectively). By contrast, female patients had a higher prevalence of diabetic neuropathy, hypertension and dyslipidaemia. Compared to men, there was a trend for slightly higher baseline HbA1c and FPG levels in women (Table 2).
Glycaemic Control
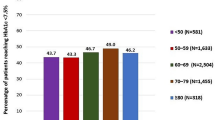
The LS mean (95% CI) reduction in HbA1c from baseline to week 24 was − 1.17% (− 1.21 to − 1.13) in men and − 1.07% (− 1.11 to − 1.02) in women, with a LS mean difference between men and women of − 0.10% (95% CI − 0.15 to − 0.05; p < 0.0001). The decrease in HbA1c in both gender subgroups happened mainly in the first 12 weeks of Gla-300 treatment and continued afterwards, as reported in Table 3. Moreover, at week 24, more men than women achieved HbA1c targets of < 7.0% (p < 0.0001; chi-square), < 7.5% and < 8.0% (Fig. 1).
The LS mean (95% CI) decrease in FPG from baseline to week 24 was − 40.32 mg/dl (− 42.84 to − 37.80) in men and − 36.00 mg/dl (− 38.65 to − 33.36) in women, resulting in a LS mean difference between men and women of − 4.32 mg/dl (95% CI − 7.48 to − 1.16; p = 0.0073). In line with changes in HbA1c, the reduction in FPG in both gender subgroups happened mainly in the first 12 weeks and continued afterwards (Table 4).
Hypoglycaemic Events
During the 24-week Gla-300 treatment period, 10.7% of men and 10.6% of women reported at least one episode of any-time-of-the-day hypoglycaemia of any type. Nocturnal hypoglycaemia of any type was reported by 2.9% of men and 2.1% of women. Incidence and event rates for symptomatic and severe any-time-of-the-day hypoglycaemia as well as symptomatic and severe nocturnal hypoglycaemia were overall low, with no notable differences between men and women (Table 5).
Insulin Dose and Body Weight
Mean ± SD Gla-300 dose increased from 0.29 ± 0.18 and 0.31 ± 0.18 U/kg/day at baseline to 0.40 ± 0.20 and 0.41 ± 0.20 U/kg/day at week 12 in men and women, respectively. Changes in the daily dose of Gla-300 remained comparable in both gender subgroups throughout the 24-week treatment period despite slightly lower levels at week 24 in men (0.36 ± 0.20) than in women (0.40 ± 0.21 U/kg/day).
The mean ± SD change in body weight from baseline to week 12 and week 24 of Gla-300 treatment was marginal in both gender subgroups: 0.12 ± 2.83 and 0.08 ± 4.21 kg for men and − 0.08 ± 2.32 and − 0.06 ± 3.64 kg for women, respectively.
Discussion
The REALI pooled analysis revealed that despite some differences in baseline demographic and clinical characteristics, both men and women with inadequately controlled T2DM achieved clinically important improvements in glycaemic control after Gla-300 treatment initiation, without notable gender differences in reported hypoglycaemia event rates or incidence, body weight change and insulin dose change. However, male gender was associated with a statistically significantly greater reduction in HbA1c and FPG from baseline compared with female gender, and women were also less likely than men to achieve HbA1c target levels after 6 months of treatment, independently of different HbA1c target thresholds. The LS mean differences in HbA1c and FPG reductions between the gender subgroups were however only − 0.10% (95% CI − 0.15 to − 0.05) and − 4.32 (95% CI − 7.48 to − 1.16) mg/dl, respectively, which renders the clinical relevance of these findings uncertain.
To date, studies on gender differences in glycaemic control have shown inconsistent results. In a 2013 pooled analysis [33] of nine RCTs, evaluating treatment outcomes with Gla-100 versus an active comparator treatment (i.e., neutral protamine Hagedorn [NPH] insulin, insulin lispro, premixed insulin, oral antidiabetic agents, dietary intervention) in 2938 patients with T2DM (1651 men and 1287 women), Gla-100-treated men were more likely to achieve HbA1c levels ≤ 7.0% than women after 24 weeks of treatment (60.8% versus 54.3%; p = 0.016). Gla-100-treated men also experienced a 0.07 percentage point greater reduction in HbA1c levels from baseline compared with women that was also statistically significant (p = 0.037) but clinically modest. Interestingly, the FPG reduction was 3.1 mg/dl greater in female than in male patients [33]. Likewise, in another patient-level pooled analysis [1] of six RCTs of Gla-100 versus NPH insulin administered for 24–36 weeks in 2600 insulin-naïve, inadequately controlled patients with T2DM (1349 men and 1251 women), significantly more men than women achieved the HbA1c target level of < 7.0% during either insulin treatment (33.0% versus 26.5%; p < 0.001). A statistically significantly greater HbA1c reduction was also observed in men than in women (− 1.36 versus − 1.22; p = 0.002); however, the difference between the genders in HbA1c reduction was clinically modest. Reductions in FPG levels were, by contrast, significantly greater in women (− 4.33 versus − 3.93 mmol/l; p = 0.009) [1]. On the other hand, a population-based, cross-sectional study from the US conducted in 1178 insulin-treated patients with T2DM identified female sex as a predictor of good glycaemic control [34]. In another retrospective, population-based study from the UK in 6032 patients with T2DM who initiated insulin therapy, gender was not a significant predictor of insulin responders who were defined as having an HbA1c < 7.5% and/or HbA1c reduction by > 1% at 12 months post-insulin initiation [35]. This inconsistency between the different studies may be partly attributed to different population characteristics and study settings, or probably to a random chance observation of a significant difference in response to insulin therapy.
In REALI, similar rates of hypoglycaemia occurring at any time of the day or during the night were reported in both gender subgroups. In the two aforementioned pooled analyses [1, 33], significantly higher annual rates of symptomatic and severe hypoglycaemia occurring during the night or at any time of the day were found among female patients. Such differences in the risk of hypoglycaemia between REALI and the two previous pooled analyses [1, 33] could be related, at least in part, to different baseline population characteristics as well as patient differences in self-monitoring of blood glucose. The smoother and more even pharmacokinetic and pharmacodynamic profiles as well as the low within-day variability of Gla-300 used in REALI compared to Gla-100 and the other first-generation insulins used once to twice daily in the aforementioned pooled analyses may have also partially contributed to the overall low and similar rates of hypoglycaemia reported in both gender subgroups of REALI [14]. Of note, REALI is, to the best of our knowledge, the first pooled analysis assessing gender differences in glycaemic and safety outcomes following Gla-300 treatment initiation. Gla-300 had a weight neutral effect for both men and women, suggesting that weight gain is not an issue of concern when initiating Gla-300 in either gender. Similarly, the Gla-300 daily dose increased during the first 12-week treatment and remained relatively stable up to 24 weeks in both men and women. These safety findings are key factors that influence patients’ adherence and quality of life, regardless of their gender.
Previous studies have reported differences in the prevalence of several cardiovascular risk factors such as overweight/obesity, hypertension and dyslipidaemia between men and women [2, 36]. Consistent with REALI in which female patients had a higher prevalence of hypertension and dyslipidaemia, a 2006 meta-analysis [37] of 37 prospective cohort studies found that women with diabetes not only have significantly higher blood pressure and lipid levels than men with diabetes, but that the difference in the levels among people with and without diabetes was significantly greater in women than it was in men [37]. In REALI, a history of MI was substantially more common in men compared to women. This is an expected finding, since the prevalence of symptomatic or severe PAD seems to be higher among men than women in the general population [38]. By contrast, asymptomatic PAD has been reported to be more frequent in women than in men, which often leads to delayed diagnosis and under-recognition of PAD in women [39]. Regarding MI, its risk has been estimated to be, on average, three times higher in men than in women [40].
The causes of the gender differences in the prevalence of cardiovascular risk factors are not completely understood. Gender differences in body anthropometry and storage patterns of adipose tissue might partly explain the differences between men and women in the burden and complications of T2DM [6, 8]. The preferential deposition of excess fat in visceral and ectopic tissues in men could lead to a faster transition to insulin resistance and diabetes, whereas women may need to gain more weight and related metabolic risk factors may need to worsen to a greater extent than in men to reach the same levels of visceral and ectopic fat that are required to develop insulin resistance and T2DM [8]. In addition, a sex-stratified Mendelian randomisation study found that the genetic risk of T2DM increased the odds of cardiovascular diseases such as coronary heart disease for both genders (odds ratio of 1.13 [95% CI 1.08–1.18]) and 1.21 [1.17–1.26] per 1-log unit increase in odds of T2DM in women and men, respectively) [41]. These findings suggest to grant equal priority in both genders for cardiovascular disease prevention in the management of T2DM [41]. There is currently a dearth of data regarding the role of Gla-300 in men and women with a variable cardiovascular risk factor presentation, despite the fact that Gla-300 has shown efficacy and safety across a broad spectrum of T2DM populations, including patients with cardiovascular risk factors such as chronic kidney disease and advanced age [14, 42]. Nevertheless, further research is needed to evaluate Gla-300 in high-risk T2DM populations and to specifically assess the impact of Gla-300 therapy on cardiovascular outcomes and on endpoints such as micro- and macrovascular complications [14, 43].
The present analysis has several strengths including the large sample size, the prospective nature of the evaluated trials, and the inclusion of adequate and balanced numbers of men and women allowing for a robust evaluation of the impact of gender on treatment outcomes in T2DM. In addition, the REALI analysis applied standardised endpoint definitions to reduce study-specific differences. There are also limitations to the current pooled analysis, such as the combination of interventional and non-interventional study data, which could increase the heterogeneity of the findings. However, this is unlikely, as the two included interventional studies (Take Control [22] and ITAS [23, 29]) are phase IV, single-drug, two-arm RCTs, with randomisation performed according to Gla-300 titration approach, i.e., patient-managed versus physician-managed titration. Another limitation may be the presence of a potential reporting bias, which is inherent to observational studies and which could result in under- or overreporting of adverse events including hypoglycaemia by the participating physicians involved in diabetes care and by the patients. Nevertheless, REALI adds great value to the literature on gender differences in T2DM treatment outcomes and supports the need to individualise diabetes therapy taking into account all specificities including but not limited to gender differences. REALI data further support the initiation of Gla-300 in both women and men with inadequately controlled T2DM.
Conclusions
Both men and women with uncontrolled T2DM achieved clinically important improvements in glycaemic control after Gla-300 treatment initiation, without notable gender differences in reported hypoglycaemia event rates or incidence, body weight and insulin dose changes. However, female patients treated with Gla-300 over a 24-week period were less likely to achieve HbA1c target levels and had statistically significantly lower, although clinically modest, reductions in HbA1c and FPG than male patients. In any case, adopting an individualised treatment approach that considers different patient characteristics, including gender, may add value in the management of patients with T2DM.
References
Kautzky-Willer A, Kosi L, Lin J, Mihaljevic R. Gender-based differences in glycaemic control and hypoglycaemia prevalence in patients with type 2 diabetes: results from patient-level pooled data of six randomized controlled trials. Diabetes Obes Metab. 2015;17(6):533–40.
Choe SA, Kim JY, Ro YS, Cho SI. Women are less likely than men to achieve optimal glycemic control after 1 year of treatment: a multi-level analysis of a Korean primary care cohort. PLoS ONE. 2018;13(5):e0196719.
Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. 2018;187:20–3.
Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG, Reusch JEB. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia. 2019;62(10):1761–72.
Tramunt B, Smati S, Grandgeorge N, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63(3):453–61.
Duarte FG, da Silva Moreira S, Almeida MCC, et al. Sex differences and correlates of poor glycaemic control in type 2 diabetes: a cross-sectional study in Brazil and Venezuela. BMJ Open. 2019;9(3):e023401.
International Diabetes Federation. IDF Diabetes Atlas: Ninth edition 2019. 2019. https://www.diabetesatlas.org/upload/resources/2019/IDF_Atlas_9th_Edition_2019.pdf. Accessed Sept 08, 2020.
de Ritter R, de Jong M, Vos RC, et al. Sex differences in the risk of vascular disease associated with diabetes. Biol Sex Differ. 2020;11(1):1.
Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(Suppl 1):60–75.
Arnetz L, Ekberg NR, Alvarsson M. Sex differences in type 2 diabetes: focus on disease course and outcomes. Diabetes Metab Syndr Obes. 2014;7:409–20.
Misra R, Lager J. Ethnic and gender differences in psychosocial factors, glycemic control, and quality of life among adult type 2 diabetic patients. J Diabetes Complicat. 2009;23(1):54–64.
Siddiqui MA, Khan MF, Carline TE. Gender differences in living with diabetes mellitus. Mater Sociomed. 2013;25(2):140–2.
Rossi MC, Cristofaro MR, Gentile S, et al. Sex disparities in the quality of diabetes care: biological and cultural factors may play a different role for different outcomes: a cross-sectional observational study from the AMD Annals initiative. Diabetes Care. 2013;36(10):3162–8.
Vargas-Uricoechea H. Efficacy and safety of insulin glargine 300 U/ml versus 100 U/ml in diabetes mellitus: a comprehensive review of the literature. J Diabetes Res. 2018;2018:2052101.
Gourdy P, Bahloul A, Boultif Z, Gouet D, Guerci B. Efficacy and safety of switching patients inadequately controlled on basal insulin to insulin glargine 300 U/ml: the TRANSITION 2 study. Diabetes Ther. 2020;11(1):147–59.
Wiesli P, Schories M. Improved glycemic control with insulin glargine 300 U/ml (Toujeo®) in patients with type 2 diabetes: real-world effectiveness in Switzerland. Diabetes Ther. 2018;9(6):2325–34.
Hidvegi T, Stella P. Effectiveness of insulin glargine U300 used as part of basal bolus therapy in people with T2DM—Toujeo 6 months real-world data from Hungary [Abstract]. Diabetes. 2018;67(Supplement 1):1049-P.
Pfohl M, Jornayvaz FR, Fritsche A, et al. Effectiveness and safety of insulin glargine 300 U/ml in insulin-naïve patients with type 2 diabetes after failure of oral therapy in a real-world setting. Diabetes Obes Metab. 2020;22(5):759–66.
Thomann R, Zechmann S, Alexander-David N, Jornayvaz FR. Real-world effectiveness of insulin glargine 300 initiation in Switzerland. Diabetes Metab Syndr Obes. 2020;13:2359–65.
Prázný M, Flekač M, Jelínek P, Mašková J. Insulin glargine 300 units/ml effectiveness in patients with T2DM uncontrolled by basal insulin in real-life settings in the Czech Republic. J Diabetes Mellitus. 2020;10(3):109–23.
Colin IM, Alexandre K, Bruhwyler J, Scheen A, Verhaegen A. Patient-reported outcomes with insulin glargine 300 U/ml in people with type 2 diabetes: the MAGE multicenter observational study. Diabetes Ther. 2020;11(8):1835–47.
Russell-Jones D, Dauchy A, Delgado E, et al. Take control: a randomized trial evaluating the efficacy and safety of self- versus physician-managed titration of insulin glargine 300 U/ml in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21(7):1615–24.
Bonadonna RC, Giaccari A, Buzzetti R, et al. Italian titration approach study (ITAS) with insulin glargine 300 U/ml in insulin-naïve type 2 diabetes: design and population. Nutr Metab Cardiovasc Dis. 2019;29(5):496–503.
Perez A, Carrasco-Sánchez FJ, González C, et al. Efficacy and safety of insulin glargine 300 U/ml (Gla-300) during hospitalization and therapy intensification at discharge in patients with insufficiently controlled type 2 diabetes: results of the phase IV COBALTA trial. BMJ Open Diabetes Res Care. 2020;8(1):e001518.
Wieringa TH, de Wit M, Twisk JW, Snoek FJ. Improved diabetes medication convenience and satisfaction in persons with type 2 diabetes after switching to insulin glargine 300 U/ml: results of the observational OPTIN-D study. BMJ Open Diabetes Res Care. 2018;6(1):e000548.
Velojic-Golubovic M, Ciric V, Dimitrijevic M, et al. Clinical benefit of insulin glargine 300 U/ml among patients with type 2 diabetes mellitus previously uncontrolled on basal or premixed insulin in Serbia: a prospective, observational, single-arm, multicentre, real-world study. Diabetes Ther. 2021;12(7):2049–58.
Stegaru D, Nicodim S, Vladu D, et al. Effectiveness and safety of insulin glargine Gla-300 in insulin-naïve type 2 diabetes subjects in a real-life setting-the GOAL_RO trial. Ann Transl Med. 2021;9(2):105.
Kamenov Z, Pehlivanova V, Kuneva T, et al. Real-world effectiveness and safety of insulin glargine 300 U/ml in patients with T2D uncontrolled on NPH or premixed insulins as part of routine clinical practice in Bulgaria: ToUPGRADE study. Diabetes Ther. 2021;12(3):913–30.
Bonadonna RC, Giaccari A, Buzzetti R, et al. Comparable efficacy with similarly low risk of hypoglycaemia in patient- vs physician-managed basal insulin initiation and titration in insulin-naïve type 2 diabetic subjects: the Italian Titration Approach Study. Diabetes Metab Res Rev. 2020;36(6):e3304.
Freemantle N, Bonadonna RC, Gourdy P, et al. Rationale and methodology for a European pooled analysis of postmarketing interventional and observational studies of insulin glargine 300 U/ml in diabetes: protocol of REALI project. BMJ Open. 2020;10(4):e033659.
European Medicines Agency. Toujeo: Summary of Product Characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/toujeo-epar-product-information_en.pdf. Accessed Sept 08. 2020.
Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–95.
McGill JB, Vlajnic A, Knutsen PG, Recklein C, Rimler M, Fisher SJ. Effect of gender on treatment outcomes in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2013;102(3):167–74.
Nichols GA, Hillier TA, Javor K, Brown JB. Predictors of glycemic control in insulin-using adults with type 2 diabetes. Diabetes Care. 2000;23(3):273–7.
Owen V, Seetho I, Idris I. Predictors of responders to insulin therapy at 1 year among adults with type 2 diabetes. Diabetes Obes Metab. 2010;12(10):865–70.
Rivellese AA, Riccardi G, Vaccaro O. Cardiovascular risk in women with diabetes. Nutr Metab Cardiovasc Dis. 2010;20(6):474–80.
Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–8.
Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–26.
Schramm K, Rochon PJ. Gender Differences in Peripheral Vascular Disease. Semin Intervent Radiol. 2018;35(1):9–16.
Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ. 2018;363:k4247.
Peters TM, Holmes MV, Richards JB, et al. Sex differences in the risk of coronary heart disease associated with type 2 diabetes: a Mendelian randomization analysis. Diabetes Care. 2021;44(2):556–62.
Hirose T, Odawara M, Matsuhisa M, et al. Risk of hypoglycemia in Japanese people with type 2 diabetes mellitus who initiated or switched to insulin glargine 300 U/ml: a subgroup analysis of 12-month post-marketing surveillance study (X-STAR study). Diabetes Res Clin Pract. 2021;172:108647.
Chang CH, Chuang LM. Cardiovascular safety of long-acting insulin analogs in type 2 diabetes patients: Is there a better basal insulin? J Diabetes Investig. 2018;9(4):728–30.
Acknowledgements
Funding
This study was funded by Sanofi (Paris, France). The journal’s Rapid Service Fee was also funded by Sanofi (Paris, France).
Medical Writing Support
Medical writing support in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) was provided by Thomas Rohban, MD, and Magalie El Hajj, PharmD, of Partner 4 Health (Paris, France) and was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
All authors contributed to the project design and the analysis plan. Celine Mauquoi performed the statistical analysis of the data. All authors were involved in the interpretation of the data, writing and reviewing drafts of the manuscript, and approved the final version for submission.
Prior Presentation
Preliminary results were presented at the 55th Annual Meeting of the European Association for the Study of Diabetes, 16–20 September 2019, Barcelona, Spain.
Disclosures
Pierre Gourdy has received advisory board and speaker honoraria from Abbott, Amgen, AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Mundipharma, Organon, Sanofi, and Servier. Riccardo C. Bonadonna has served on the speaker bureau for Sanofi, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca and Janssen, and has served on the advisory panel for Merck Sharp & Dohme, Eli Lilly, Sanofi and Johnson & Johnson. Nick Freemantle has received research support and acted as a consultant for Allergan, Ipsen, Sanofi, AstraZeneca and Novartis. Didac Mauricio has acted as a consultant and has served on the speaker bureau for Almirall, Ascensia, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Eli Lilly, Ferrer, Janssen, Menarini, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi. Dirk Müller-Wieland has acted as a consultant and has served on the speaker bureau for Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Lilly, Merck Sharp & Dohme, Novo Nordisk and Sanofi. Gregory Bigot is an IVIDATA employee. Celine Mauquoi is an IDDI employee, and has acted as a biostatistics contractor for Sanofi. Mireille Bonnemaire and Alice Ciocca are Sanofi employees.
Compliance with Ethics Guidelines
This analysis did not involve primary data collection by the authors, and all analysed data were anonymised; consequently, separate ethical approval was not required. Protocols for all included studies were approved by the appropriate ethics committees, and the studies were conducted according to Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gourdy, P., Bonadonna, R.C., Freemantle, N. et al. Does Gender Influence the Effectiveness and Safety of Insulin Glargine 300 U/ml in Patients with Uncontrolled Type 2 Diabetes? Results from the REALI European Pooled Analysis. Diabetes Ther 13, 57–73 (2022). https://doi.org/10.1007/s13300-021-01179-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01179-8