Abstract
Introduction
The PRONTO-T1D study evaluated the efficacy and safety of ultra rapid lispro (URLi) versus lispro in adults with type 1 diabetes mellitus. After 26 weeks of treatment, mealtime and postmeal URLi provided effective and comparable glycemic control in a prespecified subpopulation analysis of Japanese patients from PRONTO-T1D. We present the results of a 52-week study which evaluated the long-term efficacy and safety of URLi in Japanese patients.
Methods
After an 8-week lead-in period to optimize basal insulin treatment, Japanese patients were randomized to one of three treatment groups: the 52-week double-blind mealtime URLi (n = 62) or mealtime lispro (n = 59) group, respectively, or the 52-week open-label postmeal URLi (n = 46) group.
Results
At week 52, there were no statistically significant differences in change from baseline in hemoglobin A1c (HbA1c) between Japanese patients on URLi and those on lispro; the least-squares mean (LSM) treatment difference was 0.04% (95% confidence interval [CI] − 0.18, 0.25) between mealtime URLi and lispro, and 0.04% (95% CI − 0.19, 0.28) between postmeal URLi and mealtime lispro. No significant between-group differences were observed in the number of patients achieving the HbA1c target of < 7.0% (20.0, 30.5 and 16.3% of those on mealtime URLi, mealtime lispro and postmeal URLi, respectively). Daily average blood glucose levels in the 10-point self-monitored blood glucose profiles at week 52 were similar between treatments. However, compared with lispro, lower blood glucose levels were observed for the mealtime URLi group at the morning 1- and 2-h postmeal time points with LSM differences of − 32.7 mg/dL (− 1.82 mmol/L) (p = 0.005) and − 23.2 mg/dL (− 1.29 mmol/L) (p = 0.029), respectively. There were no significant treatment differences in the incidences of treatment-emergent adverse events, documented hypoglycemia and severe hypoglycemia; however, the rate of documented hypoglycemia was lower in the mealtime URLi arm compared with the lispro arm.
Conclusions
Overall glycemic control and improved postprandial glucose via self-monitoring was maintained in Japanese patients following 52 weeks of treatment with URLi versus lispro, including postmeal URLi administration.
Trial Registration
ClinicalTrials.gov: NCT03214367.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is a medical need for faster rapid-acting insulins, including those that can be administered postmeal as needed. |
In a prespecified Japanese subpopulation analysis of the PRONTO-T1D trial, the results were consistent with those of the overall population where ultra rapid lispro (URLi) demonstrated non-inferior hemoglobin A1c change from baseline compared to lispro following 26 weeks of treatment with mealtime URLi and lispro, and when URLi was administered 20 min after the meal. |
The long-term efficacy and safety of URLi in comparison to lispro was evaluated in an additional 26-week long-term maintenance period, for a total treatment period of 52 weeks, with this study thus being the first report of long-term efficacy and safety of postmeal URLi in any population. |
What was learned from the study? |
Overall glycemic control and improved postprandial glucose via self-monitoring was maintained in Japanese patients following 52 weeks of treatment with URLi administered at mealtime or postmeal versus lispro administered at mealtime. |
These observations suggest that the efficacy and safety profile of URLi is preserved during long-term treatment in Japanese patients with type 1 diabetes mellitus. |
Introduction
Glycemic control is central to the management of diabetes. However, despite significant advances in diabetes care and management, many patients fail to achieve optimal glycemic control [1], thereby increasing the risk of diabetes-related morbidity and mortality [2, 3]. It is clear that control of both fasting and postprandial hyperglycemia is crucial for achieving recommended hemoglobin A1c (HbA1c) targets in diabetes [2]. In Japan, the mean HbA1c of patients with type 1 diabetes mellitus (T1DM) is 7.8% [4], well above the recommended target of < 7.0% [2].
Prandial insulin analogs, such as lispro (Humalog; Eli Lilly and Company, Indianapolis, IN, USA), insulin aspart (NovoRapid; Novo Nordisk A/S, Bagsværd, Denmark), and insulin glulisine (Apidra; Sanofi S.A., Paris, France), have a faster onset and offset of insulin effect in comparison to regular human insulin [5]. However, these rapid-acting insulin analogs are still not able to match the speed of physiological insulin secretion [6], leaving many patients unable to achieve optimal glycemic control. Additionally, substantial burden was reported by Japanese patients with diabetes regarding the injection timing of mealtime insulin [7]. Prior to the approval of ultra rapid lispro (URLi) in Japan (Lyumjev; Eli Lilly and Company) in March 2020 [8], Fiasp® (Novo Nordisk A/S) was the only postmeal product available in Japan [9]. Hence, there is a medical need for more fast-acting insulin preparations.
URLi is a novel insulin lispro formulation containing two locally-acting excipients, citrate and treprostinil, with independent mechanisms of action that accelerate the absorption of insulin lispro. Microdoses of treprostinil in the URLi formulation enhance insulin lispro absorption by local vasodilation but are not associated with systemic effects [10]. Sodium citrate in the formulation further enhances the absorption of insulin lispro by increasing vascular permeability at the injection site [11]. URLi was developed with the aim to more closely match physiological insulin secretion in response to meals and improve postprandial glucose (PPG) control [12]. Pharmacokinetic and glucodynamic studies have demonstrated that in comparison to lispro URLi has accelerated absorption and faster onset/offset of action, with reduced PPG levels in patients with T1DM [12].
PRONTO-T1D was a multinational, prospective, randomized, phase 3 trial which evaluated the efficacy and safety of URLi versus lispro in adults with T1DM [13]. The findings of the first 26 weeks of PRONTO-T1D have been published [13]. This pivotal trial met the primary objective of non-inferior change in HbA1c from baseline through to week 26 when URLi and lispro were administered at mealtime (0–2 min before the start of a meal) in a double-blind manner, and for postmeal URLi administration (20 min after the start of a meal) in an open-label setting compared with mealtime lispro [13]. In this global trial, mealtime URLi was superior to mealtime lispro in controlling 1- and 2-h PPG excursions during a meal test at week 26. Postmeal URLi provided similar PPG control during the meal test in comparison with mealtime lispro, although it was less effective compared to mealtime URLi. Importantly, the improvement observed in glycemic control in the overall population at week 26 occurred without an increase in the risk of hypoglycemia, and URLi was well tolerated.
A prespecified exploratory analysis of 26-week data from the PRONTO-T1D trial in Japanese patients has also been published [14]. Consistent with the overall population, URLi was effective and comparable to lispro in terms of overall glycemic control when administered at mealtime or 20 min after the start of a meal in Japanese patients with T1DM [14]. Furthermore, mealtime URLi provided effective PPG control and was well tolerated in Japanese patients. An additional 26-week long-term maintenance period of the PRONTO-T1D trial was conducted to evaluate the long-term efficacy and safety of URLi (a total of 52 weeks of treatment). For the overall population, glycemic control and improved PPG via self-monitored blood glucose (SMBG) was maintained following 52 weeks of treatment with mealtime URLi versus mealtime lispro [15]. Importantly, no additional safety issues were identified during long-term treatment [15].
Here we report the long-term efficacy and safety results of mealtime URLi compared with mealtime lispro from the 52-week treatment period in Japanese patients with T1DM. In addition, we present the results of postmeal URLi versus mealtime lispro following 52 weeks of treatment in Japanese patients—the first report on long-term postmeal administration of URLi in any patient population.
Methods
Study Participants
Adults at least 18 years old with a clinical diagnosis of T1DM (based on World Health Organization classification) for at least 1 year prior to screening were eligible for participation. Participants must have been treated with a rapid-acting insulin analog for ≥ 90 days and a basal insulin for ≥ 30 days prior to screening, and have an HbA1c of 7.0–9.5% (53.0–80.3 mmol/mol). Exclusion criteria included hypoglycemia unawareness, as judged by an investigator, more than one severe hypoglycemia event requiring assistance or hyperglycemia/diabetic ketoacidosis requiring an emergency room visit or hospitalization within 6 months prior to screening. All participants provided written informed consent.
Study Design
The study design and primary results of the PRONTO-T1D trial for both the overall study population and the Japanese population have been published [13, 14]. The study design is outlined in Fig. 1. Briefly, PRONTO-T1D was a prospective, randomized, double-blind, phase 3 trial in adults with T1DM, with the aim to compare double-blind mealtime URLi to mealtime lispro with an open-label postprandial URLi treatment group compared to mealtime lispro in combination with insulin glargine or insulin degludec.
Study design. Following a 1-week screening period and an 8-week lead-in period, patients were randomized in a 4:4:3 ratio to receive mealtime URLi, mealtime lispro or postmeal URLi, in combination with insulin degludec or glargine. Following the maintenance period (week 12–26), Japanese patients in each treatment arm completed the long-term maintenance period (weeks 26–52). MMTT Mixed-meal tolerance test, N Number of patients, URLi ultra-rapid lispro
After an 8-week lead-in period to optimize basal insulin, patients were randomized to one of three treatment groups. In two of the treatment groups, URLi or lispro was administered immediately (0–2 min) prior to each meal (mealtime URLi and mealtime lispro groups, respectively) in a double-blind manner. A third open-label treatment group received URLi at 20 min after the start of the meal (postmeal URLi group). In order to assess the long-term efficacy and safety of URLi compared with lispro, patients in the overall population who were randomized to one of the two blinded arms continued with double-blind treatment for an additional 26 weeks (for a total of 52 weeks). Patients in the postmeal URLi arm completed this trial at the end of a 4-week safety follow-up period after week 26, with the exception of Japanese patients, who continued up to 52 weeks. Therefore, in contrast to the overall population, all Japanese patients randomized to any treatment group (mealtime URLi, mealtime lispro or postmeal URLi) continued with treatment for an additional 26 weeks, for a total of 52 weeks. Here we report the efficacy and safety through week 52 in a prespecified Japanese subpopulation analysis of the PRONTO-T1D trial.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and International Conference on Harmonisation’s Guideline for Good Clinical Practice. The PRONTO-T1D study protocol was reviewed and approved by institutional ethics committee at each study center.
Study Interventions and Randomization
At the start of the 8-week lead-in period, patients switched to lispro from their pre-study prandial insulin. Prandial insulin doses were not changed during the lead-in period unless required for safety reasons or to facilitate basal insulin optimization. Similarly, basal insulin was optimized during the lead-in period and not changed afterwards unless required. After the lead-in period, patients were randomly assigned to mealtime URLi (100 U/mL), mealtime lispro (100 U/mL) or postmeal URLi (100 U/mL) in a 4:4:3 randomization ratio. During the initial 12 weeks of treatment, prandial insulin was adjusted as necessary to meet target SMBG levels. During the maintenance period (weeks 12–26), prandial and basal insulin doses were only adjusted if necessary to maintain glycemic control or for safety reasons. During the long-term maintenance period (weeks 26–52), prandial and basal insulin were titrated as required to maintain or optimize glycemic control at the discretion of the investigator.
Self-Monitored Blood Glucose
Patients performed 10-point SMBG profiles prior to scheduled visits and measured blood glucose levels premeal, and at 1 and 2 h after the start of the morning, midday and evening meals and at bedtime. Additional SMBG readings were scheduled as needed for glucose self-management.
Statistical Analysis
The statistical methods were as previously described [13] and were applied to the Japanese population in a prespecified subset analyses, although statistical power was not taken into consideration, and p values are provided as a reference. Briefly, the actual HbA1c level and the change in HbA1c from baseline up to week 52 were analyzed using a mixed-effects model for repeated measurements (MMRM). Additional continuous efficacy variables and the change from baseline for these variables were also analyzed using MMRM. Safety analyses were conducted on all randomized patients who received ≥ 1 dose of investigational product. Hypoglycemia was summarized by rate and incidence of events using a negative binomial regression model and a logistic regression model, respectively.
Results
Demographics and Patient Disposition in the Japanese Subpopulation
Overall, 167 Japanese patients were randomized to the mealtime URLi (n = 62), mealtime lispro (n = 59) or postmeal URLi (n = 46) treatment groups. The majority of Japanese patients (n = 163; 97.6%) completed the study. Disposition of Japanese patients from enrollment to week 52 is outlined in the Electronic Supplementary Material (ESM). Baseline characteristics for the Japanese patients are outlined in Table 1. Overall, baseline characteristics were similar between treatment groups.
Efficacy
Hemoglobin A1c
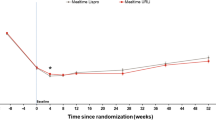
In Japanese patients, the mean HbA1c at study entry was 8.0% (63.8 mmol/mol) for the mealtime URLi group, 7.9% (62.9 mmol/mol) for the mealtime lispro group and 8.0% (63.4 mmol/mol) for the postmeal URLi group. Mean HbA1c improved during the lead-in period to a baseline value of 7.5% (58.7 mmol/mol) for the mealtime URLi group, 7.4% (57.8 mmol/mol) for the mealtime lispro group and 7.5% (58.6 mmol/mol) for the postmeal URLi group. HbA1c stabilized during the 52-week treatment period, and no statistically significant treatment differences in actual or change from baseline HbA1c were observed at weeks 26 and 52. At week 52, least-square mean (LSM) differences in HbA1c between the mealtime URLi and lispro groups were 0.04% (95% confidence interval [CI] − 0.18 to 0.25) (0.4 mmol/mol, 95% CI − 2.0 to 2.7), and LSM differences between the postmeal URLi and mealtime lispro groups were 0.04% (95% CI − 0.19 to 0.28) (0.4 mmol/mol, 95% CI − 2.1 to 3.0). Mean HbA1c levels from study entry to week 52 in Japanese patients are presented in Fig. 2.
Mean HbA1c from study entry to week 52 in Japanese patients. HbA1c levels during the lead-in period and from baseline to week 52 (% and mmol/mol) in Japanese patients treated with mealtime URLi or lispro, or postmeal URLi. Data are presented as the mean at study entry and LSM ± standard error (SE) at all other time points. CI Confidence interval, HbA1c hemoglobin A1c, LSM least squares mean
At week 52, there were no significant differences between treatment groups in terms of the number of patients achieving the HbA1c target of < 7.0% (n = 12 [20.0%], mealtime URLi group; n = 18 [30.5%], mealtime lispro group; n = 7 [16.3%], postmeal URLi group) or ≤ 6.5% (n = 8 [13.3%], mealtime URLi group; n = 9 [15.3%], mealtime lispro group; n = 1 [2.3%], postmeal URLi group).
Self-Monitored Blood Glucose
The 10-point SMBG profile at week 52 for Japanese patients in all treatment groups is outlined in Fig. 3. Overall, daily average blood glucose levels in the 10-point SMBG profiles at week 52 were similar between treatments. However, compared with the mealtime lispro group, lower blood glucose levels were observed for the mealtime URLi at the morning 1- and 2-h postmeal time points, with LSM differences of − 32.7 mg/dL (− 1.82 mmol/L) (p = 0.005) and − 23.2 mg/dL (− 1.29 mmol/L) (p = 0.029), respectively. On the other hand, compared with the mealtime URLi group, lower blood glucose levels at the midday premeal time point were observed in the mealtime lispro group, with LSM difference of 18.5 mg/dL (1.03 mmol/L) (p = 0.032). No statistically significant differences were observed between treatment groups for changes in daily mean glucose or daily mean PPG levels from baseline to week 52 in Japanese patients (Table 2), except that, compared with the mealtime lispro group, the mealtime URLi group showed a statistically significant improvement in daily mean 1-h PPG excursions (LSM difference − 17.7 mg/dL [− 0.98 mmol/L]; p = 0.028).
Ten-point self-monitored blood glucose profile at week 52 in Japanese patients. Self-monitored blood glucose profiles (mg/dL and mmol/L) following treatment with mealtime URLi, mealtime lispro or postmeal URLi at the time points of morning, midday, evening and bedtime. Data are presented as the LSM ± SE
Insulin Dose
The daily basal, bolus and total insulin dose at week 52 in Japanese patients is outlined in the ESM. Overall, daily bolus insulin doses and the ratio of prandial to total insulin dose at week 52 were similar in each treatment group, and a significantly higher basal insulin dose was observed in both URLi treatment arms compared with lispro.
Safety and Tolerability
A summary of adverse events (AEs) is outlined in Table 3. One patient death occurred in the postmeal URLi arm after week 26 and was not considered related to the study drug. Overall, the incidences of treatment-emergent AEs (TEAEs) related to the study treatment, serious AEs (SAEs) and discontinuations from the study treatment due to an AE were similar across treatment arms. The incidence of injection site reactions/pain was low and similar across treatment arms (≤ 2.2%). Average weight gain from baseline to week 52 was similar between groups: 1.0 kg in the mealtime URLi group, 1.4 kg in the mealtime lispro group and 1.3 kg in the postmeal URLi group.
The rate (events/patient/year) and incidence (%) of hypoglycemia (with or without symptoms) from week 0 to week 52 in Japanese patients is outlined in Fig. 4. The rate of documented hypoglycemia (events/patient/year) was statistically significantly lower for mealtime URLi compared with mealtime lispro in Japanese patients (blood glucose < 54 mg/dL [3.0 mmol/L]) (Fig. 4a). No significant differences were observed between groups in the incidence of documented and nocturnal hypoglycemia (Fig. 4a). The rate of severe hypoglycemia (events/patient/year) from week 0 to week 52 was low and not significantly different between treatment arms, with an aggregated rate (SE) of 0.03 (0.02) for mealtime URLi, 0.15 (0.09) for mealtime lispro and 0.04 (0.03) for postmeal URLi. There were no clinically significant treatment differences in the rate and incidence of postprandial hypoglycemia; however, in the late postprandial period (> 4 h after the meal), mealtime URLi was associated with a significant reduction in hypoglycemia compared to mealtime lispro (p = 0.006). There were no clinically significant treatment differences in the rate and incidence of postprandial hypoglycemia between postmeal URLi and mealtime lispro.
Rate and incidence of hypoglycemia (with or without symptoms) from week 0 to week 52 (blood glucose < 54 mg/dL [3.0 mmol/L]) in Japanese patients. a Rate and incidence of documented and nocturnal hypoglycemia (blood glucose < 54 mg/dL), b documented symptomatic and asymptomatic postmeal hypoglycemia (blood glucose < 54 mg/dL) . Data are presented as the LSM ± SE for event rate and LSM for incidence. RR Relative rate
Discussion
The PRONTO-T1D trial met the endpoint of non-inferior HbA1c change from baseline compared to lispro following 26 weeks of treatment in the overall population and in subpopulation of Japanese patients, as shown in previously published data [13, 14]. In study, we evaluated the long-term efficacy and safety following an additional 26-week long-term maintenance period of PRONTO-T1D (over a total of 52 weeks of treatment) in a prespecified subpopulation analysis in Japanese patients.
Overall, our findings are consistent with the initial 26-week data [14]. In comparison to mealtime lispro, treatment with URLi (both mealtime and postmeal administration) resulted in comparable long-term HbA1c control and improved PPG via self-monitoring. In addition, the long-term safety profile was similar between the URLi and lispro treatment groups. This is the first report of the long-term efficacy and safety of URLi compared to lispro in Japanese patients with T1DM. It is also the first report of the long-term efficacy and safety of postmeal URLi, as Japanese patients in PRONTO-T1D were the only patients to complete the postmeal URLi treatment arm through to 52 weeks.
Throughout the 52-week treatment period, in Japanese patients mealtime URLi and mealtime lispro were comparable in terms of HbA1c control. For both treatment arms, mean HbA1c improved during the lead-in period, and remained stable during the maintenance period (weeks 12–26) and the long-term maintenance period (weeks 26–52). At week 52, there were no statistically significant treatment differences in actual or change from baseline HbA1c between the mealtime URLi and mealtime lispro groups, with the estimated treatment difference at week 52 being consistent with that previously reported at 26 weeks [14]. The proportions of patients meeting HbA1c targets at week 52 were also similar between treatment arms. Overall, consistent mean HbA1c profiles between the mealtime URLi group and mealtime lispro group from study entry through to week 52 were observed for both the subpoulation of Japanese patients and the overall study population [15]. During the long-term maintenance period (week 26–52), mean HbA1c levels for mealtime URLi and mealtime lispro groups slightly increased in Japanese patients (7.39% to 7.46% for mealtime URLi, 7.35% to 7.42% for mealtime lispro) and in the overall population (from 7.22% to 7.47% for mealtime URLi, from 7.29% to 7.54% for mealtime lispro) [15]. The reason for this increase is unclear but may relate to compliance to diabetes treatments following behavioral changes (such as nutritional control and physical activities) when the time between visits increased after week 26.
Regarding mealtime URLi, The basal insulin dose (SE) was higher in the mealtime URLi arm compared to the mealtime lispro group: 0.29 (0.005) versus 0.26 (0.005) U/kg/day, respectively. However, the basal insulin dose for the mealtime URLi arm did not change throughout the study (0.29 U/kg/day at baseline, week 26 and week 52), and no change in SMBG was observed at the morning premeal fasting time point between week 26 and week 52. Conversely, the basal insulin dose for the mealtime lispro arm was 0.27 U/kg/day at baseline and week 26, and 0.26 U/kg/day at week 52, and a change in SMBG was observed at the morning premeal time point from 160 mg/dL at week 26 to 170 mg/dL at week 52. The change in basal insulin dose in the mealtime lispro arm may have been due to a need to maintain blood glucose control or for safety reasons.
Similarly, for the postmeal URLi treatment arm, mean HbA1c decreased during the lead-in period. However, at weeks 4 and 12, mean HbA1c in the postmeal URLi group was significantly higher than that in the mealtime lispro treatment arm. Given that patients first modified their prandial injection timing at the end of the lead-in period (i.e. at randomization) from premeal to postmeal (20 min after the start of the meal), this increase may reflect the time required by clinicians and patients to adjust to postmeal administration and glucose control. By week 26, mean HbA1c for the postmeal URLi group was still higher compared with that for the mealtime lispro group, although there was no statistically significant differences between treatment arms. An interesting observation is that mean HbA1c levels in the postmeal URLi group decreased during the long-term maintenance period (weeks 26–52) and was comparable to that observed for the other treatment arms at week 52 (7.46% for mealtime URLi, 7.42% for mealtime lispro, 7.47% for postmeal URLi). The trend from week 4 to week 52 may again be due to the time required for both practitioners and patients in this open-label arm to get accustomed and adjust to diabetes management with postmeal dosing of URLi, including diet and exercise therapy. It should be noted that this trend was not achieved by increasing bolus and/or basal insulin dose (bolus insulin 0.49 U/kg/day at 26 weeks, 0.48 U/kg/day at 52 weeks; basal insulin 0.28 U/kg/day at 26 weeks, 0.29 U/kg/day at 52 weeks). The HbA1c data at 52 weeks suggest that glycemic control may be possible using postmeal administration of URLi. However, long-term postprandial use of URLi should be considered with caution because it is generally understood that postprandial blood glucose levels with mealtime injections are lower than those with postmeal injection of insulin, with a similar direction being observed in our study.
Compared with mealtime lispro, patients receiving mealtime URLi had lower PPG following the morning meals (1- and 2-h postmeal time points) and lower daily mean 1-h glucose excursions. Differences in postmeal glucose concentrations between the URLi and lispro treatment arms were only observed after breakfast, possibly due to the dawn phenomenon, the term used to describe an early morning increase in blood glucose [16]. In addition, the SMBG values of mealtime URLi were not statistically different from those of mealtime lispro at the midday and evening postmeal time points, probably because pre-midday and pre-evening SMBG values of mealtime lispro were lower compared to those of mealtime URLi. Overall, the 10-point SMBG profiles following 52 weeks of treatment support the finding from the 26-week data, namely that it can be concluded that URLi may have superior PPG control in comparison to lispro [13]. The 10-point SMBG profile for postmeal URLi was similar to that for mealtime lispro for all time points in Japanese patients. Although PPG at 1 h after a meal was slightly higher at week 26 for postmeal URLi compared to mealtime lispro [14], it was comparable between treatment arms by week 52.
Mealtime and postmeal URLi were well tolerated by Japanese patients in this 52-week study. The safety profile and overall frequency of SAEs and discontinuations from the study due to an AE was similar between all treatment arms. The incidence of injection site reactions/pain was low and similar across treatment arms.
Importantly, adequate glycemic control in Japanese patients treated with URLi was achieved without an increase in the risk of hypoglycemia. There were no differences in the incidence of documented and nocturnal hypoglycemia (with or without symptoms) or severe hypoglycemia between treatment arms in Japanese patients through week 52 (blood glucose < 54 mg/dL [3.0 mmol/L]). Furthermore, the rate of documented hypoglycemia (events/patient/year) was significantly lower for the mealtime URLi group compared with the mealtime lispro group, consistent with the findings from week 26 [14]. In terms of postmeal hypoglycemia, there were no clinically significant treatment differences in the rate and incidence between treatment arms. However, consistent with the findings from the 26-week study [14], mealtime URLi was associated with a significant reduction in hypoglycemia compared to mealtime lispro in the late postprandial period (> 4 h after start of meal) at week 52.
The long-term findings in the current clinical trial regarding efficacy and safety of URLi following 52 weeks of treatment in Japanese patients are consistent with those observed following 26 weeks of treatment [14]. In addition, our findings suggest that long-term use of postmeal URLi may be just as effective as that of mealtime lispro without any increase in safety concerns. However, we believe that mealtime URLi should be considered as the first option, with postmeal URLi to be used only when necessary due to the general understanding that PPG is higher with postmeal dosing. Overall, our findings regarding the efficacy and safety of URLi are consistent with those observed in the overall population, at both the 26-week [13] and 52-week time points [15].
In March 2020, URLi was granted approval in Japan under the trade name Lyumjev [8]. Our findings regarding the long-term efficacy and safety of URLi are clinically important given that mealtime insulin is essential for people with T1DM throughout their life. In addition, there is a medical need for rapid-acting insulin analogs for targeted control of PPG without an increased risk of hypoglycemia and for overcoming the burden regarding current injection timing of mealtime insulin [13]. Our findings of the long-term efficacy and safety of URLi provides an additional valuable option for Japanese patients from both perspectives.
A key limitation of the study design was the use of an open-label design for the postmeal treatment arm. In addition, the small number of Japanese patients in this subpopulation analysis limited the likelihood of obtaining statistical differences in this population.
Conclusion
Overall glycemic control and improved PPG via SMBG was maintained in Japanese patients following 52 weeks of treatment with mealtime and postmeal URLi versus mealtime lispro. There were no significant treatment differences in the incidences of TEAEs and documented or severe hypoglycemia events. These observations suggest that the efficacy and safety profile of URLi is preserved during long-term treatment in Japanese patients with T1DM.
References
Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–9.
American Diabetes Association. Standards of medical care in diabetes-2019 abridged for primary care providers. Diabetes Care. 2018;42:S1.
Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16.
Japan Diabetes Clinical Data Management Study Group. 2018. https://jddm.jp/data/index-2017.html#data_03. Accessed 09 Nov 2020.
Grunberger G. Insulin analogs-are they worth it? Yes! Diabetes Care. 2014;37:1767–70.
Heinemann L, Muchmore DB. Ultrafast-acting insulins: state of the art. J Diabetes Sci Technol. 2012;6:728–42.
Ishii H, Shuichi S, Williams P, Demiya S, Aranishi T, Treuer T. Cross-sectional survey in patients with type 1 and type 2 diabetes to understand mealtime insulin unmet needs in Japan: the MINUTES-J study. Diabetes Res Clin Pract. 2020;2020:108076.
Pharmaceutical and Medical Devices Agency. Summary of investigation results. Preparations containing insulin. 2020. https://www.pmda.go.jp/files/000235075.pdf#page=4. Accessed 09 Nov 2020.
Haahr H, Heise T. Fast-acting insulin aspart: a review of its pharmacokinetic and pharmacodynamic properties and the clinical consequences. Clin Pharmacokinet. 2020;59(2):155–72. https://doi.org/10.1007/s40262-019-00834-5.
Pratt ELJ, Heilmann C, Johnson J, Landshulz W. Treprostinil causes local vasodilation, is well tolerated, and results in faster absorption of insulin lispro. Diabetes. 2017;66:975.
Moyers JSZC, Siesky AM, et al. Exploration of the mechanism of accelerated absorption for a novel insulin lispro formulation. Diabetes. 2017;2017:66.
Heise T, Linnebjerg H, Coutant D, et al. Ultra rapid lispro lowers postprandial glucose and more closely matrefches normal physiological glucose response compared to other rapid insulin analogues: a phase 1 randomized, crossover study. Diabetes Obes Metab. 2020;22:1789–98.
Klaff L, Cao D, Dellva MA, et al. Ultra rapid lispro improves postprandial glucose control compared with lispro in patients with type 1 diabetes: results from the 26-week PRONTO-T1D study. Diabetes Obes Metab. 2020;22(10):1799–1807. https://doi.org/10.1111/dom.14100.
Miura J, Imori M, Nishiyama H, Imaoka T. Ultra-rapid lispro efficacy and safety compared to Humalog(®) in Japanese patients with type 1 diabetes: PRONTO-T1D subpopulation analysis. Diabetes Ther. 2020;11:2089–104.
Bue-Valleskey J, Klaff L, Cho JI, Dellva MA, et al. Long-term efficacy and safety of ultra rapid lispro (URLi) in adults with type 1 diabetes: the PRONTO T1D extension. Diabetes Ther. 2021;12(2):569–80. https://doi.org/10.1007/s13300-020-00987-8.
Schmidt MI, Hadji-Georgopoulos A, Rendell M, Margolis S, Kowarski A. The dawn phenomenon, an early morning glucose rise: implications for diabetic intraday blood glucose variation. Diabetes Care. 1981;4(6):579–85. https://doi.org/10.2337/diacare.4.6.579.
Acknowledgements
We thank the participants and their families/caregivers, the study investigators and their staff and the PRONTO-T1D clinical trial team.
Funding
This study was sponsored by Eli Lilly and Company, IN, USA. This report, including the Journal’s Rapid Service Fee, was sponsored by Eli Lilly Japan K.K. (Kobe, Japan). All authors had full access to all data in this study and take complete responsibility for the integrity of the data and accuracy of the data analyses.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
JM was involved in data interpretation. MI was involved in study concept and design and data collection and interpretation. HN was involved in study concept and design and data analysis and interpretation. All authors were involved in drafting of the manuscript.
Medical writing, editorial, and other assistance
Medical writing support was provided by Lisa Cossens, and editorial support by Dana Schamberger, of Syneos Health and funded by Eli Lilly Japan K.K. Project management support was provided by Megumi Katoh from Eli Lilly Japan K.K.
Disclosures
Makoto Imori and Hiroshi Nishiyama are employees of Eli Lilly Japan K.K. and are minor stockholders of Eli Lilly and Company. Junnosuke Miura discloses the following relationships: receipt of speaker's fees from Taisho Pharma, Novo Nordisk, Novartis, Eli Lilly, Sanofi, Johnson & Johnson, Abbott, Terumo, Medtronic, Boehringer Ingelheim, Kyowa Kirin, Tanabe-Mitsubishi, MSD, Mylan, AstraZeneca and Astellas; consultant fees from Kowa, Kanro, Terumo, AstraZeneca and Abbott.
Compliance with ethics guidelines
The study protocol was approved by Eli Lilly and Company. Informed consent was obtained from all individual participants included in the study. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and International Conference on Harmonisation’s Guideline for Good Clinical Practice. The PRONTO-T1D study protocol was reviewed and approved by institutional ethics committee at each study center.
Data availability
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Miura, J., Nishiyama, H. & Imori, M. Long-term Efficacy and Safety of Ultra Rapid Lispro in Japanese Patients With Type 1 Diabetes: Subpopulation Analysis of the 52-Week PRONTO-T1D Study. Diabetes Ther 12, 2471–2484 (2021). https://doi.org/10.1007/s13300-021-01124-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01124-9