Abstract
Introduction
Although global studies have investigated the combination of dulaglutide with insulin in patients with type 2 diabetes mellitus (T2DM), differences in lean body mass and dulaglutide dosing can complicate the extrapolation of global study results to Japanese patients. This phase 4, randomized, placebo-controlled, double-blind, and subsequent open-label study aimed to assess the efficacy and safety of once-weekly dulaglutide 0.75 mg in combination with insulin therapy in patients with T2DM.
Methods
Patients enrolled in this multicenter study were Japanese with T2DM who had inadequate glycemic control (HbA1c 7.5–10.5%) with insulin therapy (basal insulin, premixed insulin, or basal/mealtime insulin) in combination with or without one or two oral antidiabetic agents (OADs). Patients were randomized in a 3:1 ratio to dulaglutide or placebo. The first 16 weeks was the double-blind period with stable insulin dosing, and patients taking placebo were switched to dulaglutide for an additional 36-week open-label period in which all patients took dulaglutide (52 weeks total).
Results
Patients (N = 159) were randomized to dulaglutide (n = 120) or placebo (n = 39). The least-squares (LS) mean changes from baseline in HbA1c at week 16 were dulaglutide − 1.45% and placebo 0.06%. The LS mean and 95% confidence interval for the difference were − 1.50% (− 1.73%, − 1.28%) and dulaglutide was superior to placebo. There were no significant differences between treatment groups in changes from baseline in body weight and insulin dose. The most frequently observed treatment-emergent adverse events in dulaglutide were nasopharyngitis, constipation, abdominal discomfort, nausea, and decreased appetite. The incidence rates of hypoglycemic events by week 16 were dulaglutide 42.5% and placebo 30.8% (P = 0.258).
Conclusion
Once-weekly dulaglutide 0.75 mg was superior to once-weekly placebo in glycemic control improvement and well tolerated in patients with T2DM in combination with insulin therapy with or without OADs.
Trial Registration
ClinicalTrials.gov, NCT02750410.
Funding
Eli Lilly and Company.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why Carry Out This Study? |
GLP-1 receptor agonists (such as dulaglutide) as an add-on to insulin in the treatment of type 2 diabetes mellitus (T2DM) can result in greater glycated hemoglobin A1c (HbA1c) reduction, lower incidence of hypoglycemia, and less weight gain associated with lower insulin doses versus insulin treatment alone. |
Although global studies have investigated the combination of dulaglutide with insulin in patients with T2DM, differences in lean body mass and dulaglutide dosing can complicate the extrapolation of global study results to Japanese patients. |
This study aimed to assess the efficacy and safety of once-weekly dulaglutide 0.75 mg in combination with insulin therapy with or without 1 or 2 oral antidiabetic agents in Japanese patients with T2DM. |
What Was Learned from the Study? |
In this Japanese patient population, dulaglutide 0.75 mg was superior to placebo at week 16 (P < 0.001) in reducing the HbA1c from baseline. |
There were no significant differences between treatment groups regarding changes from baseline in body weight and insulin dose. |
No new safety concerns were identified; the safety profile of dulaglutide was consistent with the safety profile of the label. |
Introduction
Insulin is one of the major therapies for type 2 diabetes mellitus (T2DM), but dosing is difficult to optimize because of hypoglycemia and body weight gain [1]. Glucagon-like peptide 1 (GLP-1) receptor agonists, which are another type of injectable antidiabetic medication, have been used recently for the treatment of T2DM in combination with insulin and/or oral antidiabetic drugs (OADs) [2,3,4]. Benefits of combining GLP-1 receptor agonists and insulin include greater glycated hemoglobin A1c (HbA1c) reduction, lower incidence of hypoglycemia, and less weight gain associated with lower insulin doses than insulin treatment alone [5, 6].
Dulaglutide (Dula) is a long-acting GLP-1 receptor agonist that mimics the effects of endogenous GLP-1 [7]. In phase 3 studies in Japanese patients with T2DM, once-weekly Dula 0.75 mg was superior to insulin glargine in a randomized, 26-week, open-label study of Dula in combination with sulfonylureas and/or biguanides [8] and noninferior to liraglutide 0.9 mg/day in a randomized monotherapy study in which Dula was compared to placebo (Plc) [9] and to liraglutide 0.9 mg/day in HbA1c changes [10]. In a nonrandomized, open-label, 52-week phase 3 safety study in Japanese patients with T2DM, once-weekly Dula 0.75 mg was well tolerated overall in combination with a single OAD (i.e., sulfonylurea, biguanide, α-glucosidase inhibitor, thiazolidinedione, or glinide) [11].
The combination of Dula and insulin has been investigated in global studies. The efficacy and safety of Dula in combination with mealtime insulin were assessed in the AWARD-4 study [12] and in combination with basal insulin in the AWARD-9 study [13]. Because of differences in lean body mass, daily insulin doses in patients with diabetes in Japan are lower than those observed in the Western population [14]. In clinical studies with Japanese populations, average daily insulin doses in patients with T2DM were 10–15 IU for basal insulin [15,16,17] and those in patients with type 1 diabetes mellitus (T1DM) or T2DM were 40–50 IU for basal/mealtime insulin [18,19,20]. It should also be noted that the approved dosage of Dula in Japan is 0.75 mg, which is half of the maximum dose used in other countries. For these reasons, it is difficult to extrapolate the results from the global studies to Japan.
This study assessed the efficacy and safety of once-weekly Dula 0.75 mg in combination with insulin therapy (i.e., basal insulin, premixed insulin, or basal/mealtime insulin) with or without one or two OADs in Japanese patients with T2DM. We hypothesized that Dula 0.75 mg would be superior to Plc regarding the change from baseline in HbA1c at week 16 in this Japanese patient population.
Methods
Study Design and Treatment
This was a phase 4, randomized, Plc-controlled, double-blind, and subsequent open-label study. The study included a 4- or 14-week screening and lead-in with or without washout period. Dipeptidyl peptidase 4 (DPP-4) inhibitors, sulfonylureas, and glinides were washed out during the screening period; all other OADs were allowed. The study also included a 16-week, double-blind, primary treatment period; a 36-week, open-label, extension treatment period; and a 4-week, safety follow-up visit (Fig. 1).
H9X-JE-GBGF study design. The study featured a 16-week double-blind primary treatment period, a 36-week open-label extension treatment period, and a 4-week safety follow-up visit. Please note that a telephone visit (T/V1; or site visit, if preferred) was scheduled at week − 12 after screening results became available. DPP-4i dipeptidyl peptidase-4 inhibitors, Dula dulaglutide, F/U follow-up, IC informed consent, n number of patients, OAD oral antidiabetic agent, Plc placebo, SU sulfonylurea, T/V telephone visit. An optional telephone visit (T/V2; or site visit if preferred) could occur between weeks − 8 and 0 or at any time during the study, as needed
Patients were randomized in a 3:1 ratio to Dula 0.75 mg or Plc via an interactive web-response system. This ratio was adopted to reduce the number of patients randomized to Plc. Randomization was stratified by insulin regimen (i.e., basal insulin, premixed insulin, or basal/mealtime insulin) and HbA1c (< 8.5% or ≥ 8.5%) at visit 2. During the 16-week primary treatment period, either Dula 0.75 mg or Plc was administered once weekly as subcutaneous injections via a single-dose pen. To preserve the blinding of the study, the treatment assignments in the double-blind period were blinded to patients and investigators until study completion. During the 36-week extension period, Dula 0.75 mg was administered to all patients once weekly as a subcutaneous injection by single-dose pen. Dosing was administered once weekly at any time of day. If a dose was missed, the missed dose was given as soon as possible after the scheduled day if there was at least 3 days until the next injection.
Unless hypoglycemia occurred, the insulin dose remained unchanged for the 16-week primary treatment period and could be adjusted to maintain target glucose values (Table S1 in the supplementary material) during the 36-week open-label period. In addition, for patients treated with one or two of the allowed/permitted OADs before visit 1, the dosage and administration of their OADs were not changed during the study period.
The study protocol was approved at each site by an institutional review board, and the study was performed in accordance with the principles of the Helsinki Declaration of 1964, as revised in 2013, concerning human and animal rights, and with the principles of Good Clinical Practice. A full list of institutional ethics committees for the participating study sites is included (Table S2 in the supplementary material). This study was approved by all participating institutions. All patients provided written informed consent before participation, in alignment with Springer’s policy concerning informed consent. The study was registered at ClinicalTrials.gov (NCT02750410).
Patients
Japanese male and female patients at least 20 years old with a diagnosis of T2DM participated in the study. Before visit 1, patients were required to be on stable doses of daily insulin for at least 3 months (± 20% versus the most commonly used dose for the period) and more than 10 units per day of either basal insulin (once or twice daily), premixed insulin (two or three times daily), or basal/mealtime insulin (four or five times daily). In addition, patients had to have HbA1c ≥ 7% and ≤ 10.5% at visit 1 if washing out OADs (i.e., DPP-4 inhibitors, sulfonylureas, or glinides) or ≥ 7.5% and ≤ 10.5% at visit 1 if not washing out OADs, and all patients had to have HbA1c ≥ 7.5% and ≤ 10.5% at visit 2. Lastly, patients had to have stable weight (defined as ± 5% at least 3 months before visit 1) and a body mass index of 18.5–35 kg/m2.
Key exclusion criteria were a diagnosis of T1DM, treatment with a GLP-1 receptor agonist and/or weight loss-promoting drugs within 3 months before visit 1, at least one episode of severe hypoglycemia diabetic ketoacidosis within 6 months before visit 1, and a history of any other condition which, in the opinion of the investigator, could prevent the patient from following and completing the protocol.
Study Assessments
The primary efficacy measure was change from baseline in HbA1c at week 16. Secondary efficacy measures included change in HbA1c from baseline through week 52, percentage of patients achieving HbA1c < 7% or ≤ 6.5% targets at weeks 16 and 52, and changes from baseline at week 16 and week 52 in fasting blood glucose (FBG), 7-point self-monitored blood glucose (SMBG) profiles, body weight, and insulin dose. Health outcomes measures included Diabetes Treatment Satisfaction Questionnaire Status (DTSQs) [21] and Diabetes Therapy Related-Quality of Life (DTR-QOL) [22]. Safety measures included treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), discontinuations, adverse events of special interest (i.e., allergic/hypersensitivity reactions, cardiovascular events, pancreatitis, and any associated with the thyroid gland), hypoglycemic episodes [symptomatic or asymptomatic (SMBG < 70 mg/dL)], and laboratory measures.
Statistical Analyses
Primary and secondary efficacy analyses were performed on the full analysis set population (all patients who received at least one dose of study treatment and have at least one measurement of HbA1c after study treatment). For the primary endpoint, a mixed-model repeated measures (MMRM) analysis for HbA1c change from baseline to week 16 using restricted maximum likelihood with treatment, insulin regimen (i.e., basal insulin, premixed insulin, or basal/mealtime insulin), visit, and treatment-by-visit as fixed effects, baseline HbA1c as a covariate, and patient as a random effect was employed. For secondary efficacy analyses, analysis of patients achieving HbA1c levels < 7% or ≤ 6.5% was based on repeated measures logistic regression with generalized linear mixed models, which included treatment, insulin regimen, visit, and treatment-by-visit interaction as fixed effects and baseline HbA1c as a covariate.
Secondary efficacy measures that were continuous variables (i.e., changes from baseline) other than HbA1c (i.e., body weight and FBG) were analyzed using MMRM on the full analysis set population. An MMRM model similar to that used for the primary endpoint was used. Analyses of covariance models were used for SMBG and health outcomes measures. Treatment comparisons of incidence of TEAEs and hypoglycemia were based on Fisher’s exact test.
A sample size of approximately 160 patients was needed to show that Dula was superior to Plc with greater than 99% power, assuming a treatment difference of 1% in HbA1c reduction, standard deviation of 1%, and dropout rate of 15%.
Results
Patient Disposition and Baseline Demographics
This study enrolled and followed patients between September 2016 and June 2018 at 26 study sites in Japan. A total of 192 patients with T2DM were screened, and 159 patients were randomized and treated (Plc, n = 39 and Dula, n = 120). Of those 159 patients, Plc/Dula 31/39 patients (79.5%) and Dula/Dula 111/120 patients (92.5%) completed 52 weeks (Fig. S1 in the supplementary material). The most frequent reasons for discontinuation were adverse events (AEs) (Dula/Dula, n = 5, Plc/Dula, n = 7) and patient decision (Dula/Dula, n = 3, Plc/Dula, n = 1).
Baseline demographic and baseline disease characteristics are shown in Table 1. Patients were predominately male (61.6%) with a mean age of 59.3 years. Mean duration of diabetes was 17 years, baseline HbA1c was 8.53%, and FBG was 161.8 mg/dL. Insulin regimens were evenly represented in the patient population (basal 43.4%, premixed 22%, basal/meal time 34.6%); 71.7% of patients were on OAD therapy before the study. Lastly, most patients (60.4%) had an estimated glomerular filtration rate categorized as stage G2 (≥ 60 and < 90 mL/min/1.73 m2). Overall, there were no clinically significant differences in baseline parameters between the two groups.
Efficacy
Dula-treated patients had a statistically significant greater reduction from baseline in HbA1c (LS mean [SE]) compared to Plc-treated patients after 16 weeks (Dula, − 1.45% [0.06], Plc, 0.06% [0.10]; P < 0.001; Fig. 2). The LS mean and 95% confidence interval (CI) for the difference were − 1.50% (− 1.73, − 1.28). After 52 weeks, LS mean (SE) in changes from baseline in HbA1c were similar: Dula/Dula − 1.09% (0.07), Plc/Dula − 0.83% (0.13) (Fig. 2).
Mean change from baseline in HbA1c (%). Please note that LS means at each visit from restricted maximum likelihood-based mixed-model repeated measures (MMRM) model: HbA1c value = baseline value + treatment + visit + treatment × visit + insulin regimen (basal insulin, premixed insulin, or basal/mealtime insulin), in which patient was treated as a random effect. Dula/Dula dulaglutide/dulaglutide, LS least squares, Plc/Dula placebo/dulaglutide, SE standard error
The percentage of patients achieving HbA1c < 7% at week 16 was significantly higher in Dula-treated patients (47.4%) compared to Plc-treated patients (0%) (P = 0.003). After 52 weeks, the percentage of patients achieving HbA1c < 7% was similar between treatment groups: Dula/Dula-treated patients (27.9%) and Plc/Dula-treated patients (25%). Similarly, the percentage of patients achieving HbA1c ≤ 6.5% at week 16 was higher in Dula-treated patients (25%) compared to those receiving Plc (0%). After 52 weeks, the percentage of patients achieving HbA1c ≤ 6.5% was similar between treatment groups: Dula/Dula-treated patients (10.8%) and Plc/Dula-treated patients (8.8%).
Dula-treated patients had a statistically significantly greater reduction from baseline in FBG (LS mean [SE]) compared to Plc-treated patients after 16 weeks (Dula − 34.2 mg/dL [2.8], Plc − 4.1 mg/dL [4.9]; P < 0.001). The LS mean and 95% CI for the difference were − 30.1 mg/dL (− 41.4, − 18.9). After 52 weeks, LS mean (SE) in changes from baseline in FBG were − 29.6 mg/dL (3.4) in Dula/Dula-treated patients and − 19.1 mg/dL (6.3) in Plc/Dula-treated patients. Additionally, at week 16, LS mean reductions (from baseline) in 7-point SMBG profiles were significantly greater in Dula-treated patients compared to Plc-treated patients at all time points (Fig. S2 in the supplementary material). No statistically significant differences between treatment groups in change from baseline body weight (LS mean [SE]) were observed at week 16 (Dula − 0.20 kg [0.17], Plc − 0.30 kg [0.30]; P = 0.776). The LS mean and 95% CI for the difference were − 0.10 kg (− 0.59, − 0.78). At week 52, changes from baseline body weight (LS mean [SE]) were Dula/Dula − 0.10 kg [0.23] and Plc/Dula − 1.01 kg [0.40]. There were no significant differences between treatment groups in mean change from baseline total insulin doses (LS mean [SE]) at week 16 (Dula/Dula − 1.1 U [3.1], Plc/Dula − 0.3 U [1.2]; P = 0.195) (Table S3 in the supplementary material).
The DTR-QOL and DTSQs scores are shown in Fig. 3. In evaluations by visit of the full analysis set population from baseline to week 16, the DTR-QOL total score was significantly higher (preferable) in the Dula/Dula group compared to Plc/Dula group. All factors of the DTR-QOL, except hypoglycemia, were also higher among Dula/Dula-treated patients compared to Plc/Dula-treated patients. LS mean change from baseline at week 16 for each individual question of the DTR-QOL questionnaire is displayed in Table S4 and graphically in Fig. S3 in the supplementary material. In terms of DTSQs, Dula/Dula treatment was statistically favored over Plc/Dula at week 16 for the DTSQs total score (P = 0.036).
Health outcomes measures. The analysis for health outcome was an ANCOVA with treatment and insulin regimen as fixed effects and baseline value as a covariate. ANCOVA analysis of covariance, DTSQs Diabetes Treatment Satisfaction Questionnaire status, DTR-QOL Diabetes Therapy Related-Quality of Life, Dula/Dula dulaglutide/dulaglutide, LS least squares, Plc/Dula placebo/dulaglutide, SE standard error
Safety
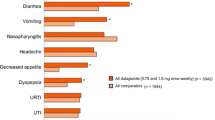
A summary of AEs through week 16 and week 56 is shown in Table 2. From baseline to week 16, a total of 84 patients (52.8%) experienced at least one TEAE. The most commonly reported TEAEs were nasopharyngitis (n = 26), constipation (n = 12), abdominal discomfort (n = 11), nausea (n = 9), and decreased appetite (n = 8). Through 16 weeks, there were no TEAEs with incidence rates that differed significantly between the treatment groups. The majority of patients reported events as mild or moderate in severity; however, three Dula-treated patients reported SAEs (hypoglycemia, osteoarthritis, and knee arthroplasty [in the same patient], as well as osteoarthritis and myositis, in one patient each). AEs leading to study drug discontinuation occurred in four Plc-treated patients (hyperglycemia [n = 2], and hypersensitivity and puncture-site pain [n = 1, each]) and four Dula-treated patients (gastrointestinal disorder, injection site reaction, liver function test increased, and myositis [n = 1, each]).
From baseline to week 56, a total of 125 patients (78.6%) experienced at least one TEAE (Table 2). The most commonly reported TEAEs were nasopharyngitis (n = 47), constipation (n = 18), abdominal discomfort (n = 16), diarrhea (n = 13), decreased appetite (n = 11), and vomiting (n = 10). Through week 56, the majority of patients reported events as mild or moderate in severity; however, a total of seven patients reported SAEs: n = 1 Plc/Dula-treated patient (depression and suicide attempt [n = 1]) and n = 6 Dula/Dula-treated patients (cataract, hypoglycemia [event occurred at 2 pm without lunch and patient was admitted to the emergency room to recover], large intestine polyp, lung adenocarcinoma, myositis, and osteoarthritis [n = 1 each]). No deaths were reported during the study.
In terms of adverse events of special interest, seven patients experienced hypersensitivity/allergic reactions (Dula n = 4 [3.3%], Plc n = 3 [7.7%]; P = 0.364) from baseline to week 16, and 12 patients experienced hypersensitivity/allergic reactions (Dula/Dula n = 8 [6.7%], Plc/Dula n = 4 [10.3%]) from baseline to week 52. One patient in the Dula/Dula group had increased lipase, but the event was deemed not to be a possible case of pancreatitis. No AEs associated with the thyroid gland were reported.
A total of 63 (39.6%) patients experienced hypoglycemia through week 16 (Dula 51 [42.5%], Plc 12 [30.8%]; P = 0.258); one case of severe hypoglycemia occurred at week 8 in a Dula-treated patient. A total of 14 (8.8%) patients experienced nocturnal hypoglycemia through week 16 (Dula/Dula 10 [8.3%], Plc/Dula 4 [10.3%]; P = 0.747). Overall, there were no clinically meaningful differences in laboratory parameters or vital signs between the Dula/Dula and Plc/Dula groups.
Discussion
In this Japanese population of patients with T2DM treated with insulin, Dula 0.75 mg was superior to Plc with respect to change from baseline in HbA1c at 16 weeks; this finding met the primary objective of the study and was consistent with the study’s original hypothesis. The difference in HbA1c between Dula 0.75 mg and Plc was − 1.50%. This was consistent with the previous phase 3 studies conducted in Japan [8, 9, 11]: a phase 3 monotherapy study in Japanese patients showed that the difference in HbA1c between Dula 0.75 mg and Plc at 26 weeks was − 1.57% [9]; and phase 3 OAD combination studies in Japanese patients in which changes from baseline in HbA1c in their respective Dula 0.75-mg groups were reported to be − 1.44% [8] and − 1.57% to − 1.69% [11].
Furthermore, there was no significant difference in body weight change between Dula and Plc at week 16 in the present study. In the previous phase 3 study in combination with a single OAD, body weight was increased in combination with thiazolidinediones, did not change in combination with sulfonylureas or glinides, and decreased in combination with biguanides or α-glucosidase inhibitors [11]. These results suggest that body weight was not decreased when Dula was added to insulin or insulin secretagogue.
In general, there were no new safety concerns. However, a single patient experienced severe hypoglycemia, which was not observed in previous Japanese phase 3 studies. As previously mentioned, the event occurred at 2 pm without lunch and the patient was admitted to the emergency room to recover.
In the AWARD-9 study, the clinical efficacy and safety of Dula 1.5 mg were assessed as an add-on to existing basal insulin therapy [23]. Changes from baseline in HbA1c were − 1.44% with Dula and − 0.67% with Plc at 28 weeks, and the difference was − 0.77% (P < 0.001). In AWARD-9, the insulin dose could be increased 4 weeks after initiating study drug (Dula 1.5 mg or Plc), and increases from baseline in daily dose of insulin were significantly smaller with Dula (13 U) versus Plc (26 U) at 28 weeks. In the current study, insulin dose was fixed during the first 16 weeks, and clinical efficacy and safety of Dula 0.75 mg and Plc were compared as an add-on therapy. Changes from baseline in HbA1c were − 1.45% with Dula and 0.06% with Plc at 16 weeks, and the difference was − 1.50% (P < 0.001). In addition, it was reported that incretin drugs such as DPP-4 inhibitors and GLP-1 receptor agonists were more effective in the Asian population [14, 24]. This may have been one of the reasons a greater HbA1c reduction was observed in Japanese patients.
In this study, we used both DTR-QOL and DTSQ to evaluate patient-reported outcomes. Despite the addition of a weekly injection, all subscales except hypoglycemia were improved in the Dula group and all subscales were worsened in the Plc group. These results suggest that Dula reduces patients’ worries and burden related to treatment for diabetes and increases treatment satisfaction. Regarding the individual questionnaire of the DTR-QOL, Dula satisfaction scores were inferior to Plc scores on Q13 [gastrointestinal symptoms (nausea, passing gas, diarrhea, abdominal pain) due to my current diabetes treatment are uncomfortable]. This was consistent with the incidence of gastrointestinal AEs reported by Dula-treated patients in this study. In terms of the items on which Dula treatment satisfaction was superior to Plc, statistically significant differences were observed for the following questions: Q7. When I eat out, it is difficult to manage my current diabetes treatment; Q8. I feel like my current diabetes treatment takes away the enjoyment of eating; Q9. With my current diabetes treatment, it is hard to curb my appetite; Q14. I am bothered by weight gain with my current diabetes treatment; Q19. I have uncomfortable symptoms due to hyperglycemia (high blood glucose); Q20. I am worried about high blood glucose; Q21. I am dissatisfied that my blood glucose is unstable (high and low); and Q26. Overall, I am satisfied with my current blood sugar control (glycemic control). Of note, Dula treatment reduced burdens on eating behaviors as well as on blood glucose control.
This study had both strengths and limitations. One strength was that it was the first study to assess the efficacy and safety of once-weekly Dula 0.75 mg in combination with insulin therapy in Japanese patients with lean body mass. Further, the Plc group showed no change in HbA1c for 16 weeks, which reflects a well-controlled study.
Some limitations of the study should also be noted. First, a treatment period of 16 weeks is relatively short for full assessments on all the key outcomes (e.g., body weight, insulin dose, hypoglycemia). Second, patients with relatively high HbA1c were enrolled in this study, so it could have been difficult to reduce the dose of insulin. If we want to focus research on how much insulin dose can be decreased (or eliminated), patients with relatively good blood glucose control should be enrolled. If insulin dose can be decreased, body weight might be decreased simultaneously.
Conclusions
In this Japanese patient population, Dula 0.75 mg was superior to Plc at week 16 (P < 0.001) in reducing the HbA1c from baseline, meeting the primary objective of the study. No new safety concerns were identified, and the safety profile of Dula was consistent with the safety profile of the label. Add-on of Dula to insulin therapy would be a useful treatment option for better blood glucose control.
References
Petznick AM. Identifying and addressing barriers to insulin acceptance and adherence in patients with type 2 diabetes mellitus. J Am Osteopathic Assoc. 2013;113(4 Suppl 2):S6–16.
Gentilella R, Pechtner V, Corcos A, Consoli A. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev. 2019;35:e3070.
Romera I, Cebrian-Cuenca A, Alvarez-Guisasola F, Gomez-Peralta F, Reviriego J. A review of practical issues on the use of glucagon-like peptide-1 receptor agonists for the management of type 2 diabetes. Diabetes Ther. 2019;10(1):5–19.
Sharma D, Verma S, Vaidya S, Kalia K, Tiwari V. Recent updates on GLP-1 agonists: current advancements and challenges. Biomed Pharmacother. 2018;108:952–62.
Balena R, Hensley IE, Miller S, Barnett AH. Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab. 2013;15:485–502.
Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228–34.
Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26:287–96.
Araki E, Inagaki N, Tanizawa Y, Oura T, Takeuchi M, Imaoka T. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open-label, phase III, non-inferiority study. Diabetes Obes Metab. 2015;17:994–1002.
Miyagawa J, Odawara M, Takamura T, Iwamoto N, Takita Y, Imaoka T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes Obes Metab. 2015;17:974–83.
Odawara M, Miyagawa J, Iwamoto N, Takita Y, Imaoka T, Takamura T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study. Diabetes Obes Metab. 2016;18:249–57.
Emoto M, Terauchi Y, Ozeki A, Oura T, Takeuchi M, Imaoka T. A 1-year safety study of dulaglutide in Japanese patients with type 2 diabetes on a single oral hypoglycemic agent: an open-label, nonrandomized, phase 3 trial. Endocr J. 2015;62:1101–14.
Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385:2057–66.
Yu M, Brunt KV, Milicevic Z, Varnado O, Boye KS. Patient-reported outcomes in patients with type 2 diabetes treated with dulaglutide added to titrated insulin glargine (AWARD-9). Clin Ther. 2017;39:2284–95.
Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:900–9.
Inagaki N, Atsumi Y, Oura T, Saito H, Imaoka T. Efficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26-week, randomized, open-label, parallel-group, multicenter, noninferiority study. Clin Ther. 2012;34:1892–908.e1.
Kawamori R, Iwamoto Y, Kadowaki T, Iwasaki M. Efficacy and safety of insulin glargine in concurrent use with oral hypoglycemic agents for the treatment of type 2 diabetic patients. Rinsyoiyaku. 2003;19:445–64.
Odawara M, Ohtani T, Kadowaki T. Dosing of insulin glargine to achieve the treatment target in Japanese type 2 diabetes on a basal supported oral therapy regimen in real life: ALOHA study subanalysis. Diabetes Technol Ther. 2012;14:635–43.
Iwamoto Y, Clauson P, Nishida T, Kaku K. Insulin degludec in Japanese patients with type 1 diabetes mellitus: a randomized controlled trial. J Diabetes Investig. 2013;4:62–8.
Kawamori R, Iwamoto Y, Kadowaki T, Iwasaki M. Comparison of efficacy between insulin glargine and NPH human insulin in type 1 diabetes patients undergoing intensive insulin treatment. Rinsyoiyaku. 2003;19:423–44.
Kobayashi M, Iwamoto Y, Kaku K, Kawamori R, Tajima N. 48-week randomized multicenter open-label parallel group phase 3 trial to compare insulin detemir and NPH insulin efficacy and safety in subjects with insulin requiring diabetes mellitus in a basal-bolus regimen. J Jpn Diabetes Soc. 2007;50(9):649–63.
Bradley C, Gamsu DS. Guidelines for encouraging psychological well-being: report of a working group of the World Health Organization Regional Office for Europe and International Diabetes Federation European Region St Vincent Declaration Action Programme for Diabetes. Diabet Med. 1994;11:510–6.
Ishii H. Development and psychometric validation of the Diabetes Therapy-Related QOL (DTR-QOL) questionnaire. J Med Econ. 2012;15:556–63.
Pozzilli P, Norwood P, Jodar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab. 2017;19:1024–31.
Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabologia. 2013;56:696–708.
Acknowledgements
We thank the patients who participated in the study, their families, and the investigators and study personnel.
Funding
This work and the Rapid Service Fee were supported by Eli Lilly and Company. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Shannon E. Gardell and Regina E. Burris of Syneos Health, LLC. Support for this assistance was funded by Eli Lilly Japan K.K. Additional assistance was provided by Aki Yoshikawa of Eli Lilly Japan K.K.
Disclosures
Masakazu Takeuchi is a full-time employee of Eli Lilly Japan K.K. and minority holder of company stock. Tomonori Oura is a full-time employee of Eli Lilly Japan K.K. and minority holder of company stock. Yukiko Onishi reports lecturer honoraria from Ono Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., and MSD K.K. as well as travel expenses and gifts from Novo Nordisk Pharma Ltd. Hitoshi Ishii reports lecturer honoraria from Novo Nordisk Pharma Ltd., MSD K.K., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Eli Lilly Japan K.K., and Sanofi K.K.
Compliance with Ethics Guidelines
The study protocol was reviewed and approved by the independent ethics committee/institutional review board at each participating center, and the study was performed in accordance with the principles of the Helsinki Declaration of 1964, as revised in 2013, concerning human and animal rights, and with the principles of Good Clinical Practice. All patients provided written informed consent before participation, in alignment with Springer’s policy concerning informed consent.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at http://www.vivli.org.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10099364.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ishii, H., Onishi, Y., Oura, T. et al. Once-Weekly Dulaglutide with Insulin Therapy for Type 2 Diabetes: Efficacy and Safety Results from a Phase 4, Randomized, Placebo-Controlled Study. Diabetes Ther 11, 133–145 (2020). https://doi.org/10.1007/s13300-019-00726-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-00726-8