Abstract
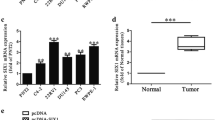
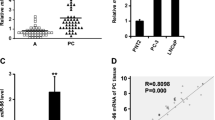
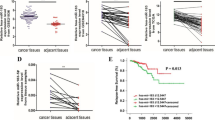
The biological role of miR-26a involved in the carcinogenesis of prostate cancer (PC) has been controversial. Besides, the underlying mechanism by which miR-26a plays a role in PC has been unclear. To investigate the role of miR-26a-5p in the PC, miR-26a-5p was detected and statistically analyzed in clinical PC tissues and a panel of PC cell lines. Using bioinformatics analysis, we found that serpine1 messenger RNA (mRNA) binding protein 1 (SERBP1) was a potential downstream target of miR-26a-5p. Using luciferase reporter and western blot, we identified that miR-26a-5p negatively regulated SERBP1 on the PC cell line level. It was confirmed that miR-26a-5p was markedly downregulated in PC tissues compared with normal controls whose reduced expression was significantly associated with metastasis and poor overall prognosis and found that miR-26a-5p was able to prevent proliferation and motility of PC cells in vitro. Additionally, SERBP1 was identified as a downstream target of miR-26a-5p. Moreover, it was observed that SERBP1 was markedly upregulated in prostate cancer tissues and was significantly associated with tissue metastasis and Gleason score. Taken together, our results for the first time demonstrate that the loss of miR-26a-5p promotes proliferation, migration, and invasion through targeting SERBP1 in PC, supporting the tumor-suppressing role of miR-26a-5p in PC.






Similar content being viewed by others
References
Hu Y, Zhao Q, Rao J, Deng H, Yuan H, Xu B. Longitudinal trends in prostate cancer incidence, mortality, and survival of patients from two Shanghai City districts: a retrospective population-based cohort study, 2000-2009. BMC Public Health. 2014;14(1):356.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Deng J, He M, Chen L, Chen C, Zheng J, Cai Z. The loss of miR-26a-mediated post-transcriptional regulation of cyclin E2 in pancreatic cancer cell proliferation and decreased patient survival. PLoS One. 2013;8(10):e76450.
Gao J, Li L, Wu M, Liu M, Xie X, Guo J, Tang H, Xie X. MiR-26a inhibits proliferation and migration of breast cancer through repression of MCL-1. PLoS One. 2013;8(6):e65138.
Ichikawa T, Sato F, Terasawa K, Tsuchiya S, Toi M, Tsujimoto G, Shimizu K. Trastuzumab produces therapeutic actions by upregulating miR-26a and miR-30b in breast cancer cells. PLoS One. 2012;7(2):e31422.
Jansen MP, Reijm EA, Sieuwerts AM, Ruigrok-Ritstier K, Look MP, Rodriguez-Gonzalez FG, Heine AA, Martens JW, Sleijfer S, Foekens JA, et al. High miR-26a and low CDC2 levels associate with decreased EZH2 expression and with favorable outcome on tamoxifen in metastatic breast cancer. Breast Cancer Res Treat. 2012;133(3):937–47.
Zhang B, Liu XX, He JR, Zhou CX, Guo M, He M, Li MF, Chen GQ, Zhao Q. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011;32(1):2–9.
Yu L, Lu J, Zhang B, Liu X, Wang L, Li SY, Peng XH, Xu X, Tian WD, Li XP. miR-26a inhibits invasion and metastasis of nasopharyngeal cancer by targeting EZH2. Oncol Lett. 2013;5(4):1223–8.
Lin Y, Chen H, Hu Z, Mao Y, Xu X, Zhu Y, Wu J, Li S, Mao Q, Zheng X, et al. miR-26a inhibits proliferation and motility in bladder cancer by targeting HMGA1. FEBS Lett. 2013;587(15):2467–73.
Deng M, Tang HL, XH L, Liu MY, XM L, YX G, Liu JF, He ZM. miR-26a suppresses tumor growth and metastasis by targeting FGF9 in gastric cancer. PLoS One. 2013;8(8):e72662.
Zhang Y, Zhang B, Zhang A, Li X, Liu J, Zhao J, Zhao Y, Gao J, Fang D, Rao Z. IL-6 upregulation contributes to the reduction of miR-26a expression in hepatocellular carcinoma cells. Braz J Med Biol Res. 2013;46(1):32–8.
Guo P, Lan J, Ge J, Nie Q, Guo L, Qiu Y, Mao Q. MiR-26a enhances the radiosensitivity of glioblastoma multiforme cells through targeting of ataxia-telangiectasia mutated. Exp Cell Res. 2014;320(2):200–8.
Liu B, Wu X, Wang C, Liu Y, Zhou Q, Xu K. MiR-26a enhances metastasis potential of lung cancer cells via AKT pathway by targeting PTEN. Biochim Biophys Acta. 2012;1822(11):1692–704.
Shen W, Song M, Liu J, Qiu G, Li T, Hu Y, Liu H. MiR-26a promotes ovarian cancer proliferation and tumorigenesis. PLoS One. 2014;9(1):e86871.
Tian L, Fang YX, Xue JL, Chen JZ. Four microRNAs promote prostate cell proliferation with regulation of PTEN and its downstream signals in vitro. PLoS One. 2013;8(9):e75885.
Zhao S, Ye X, Xiao L, Lian X, Feng Y, Li F, Li L. MiR-26a inhibits prostate cancer progression by repression of Wnt5a. Tumour Biol. 2014;35(10):9725–33.
Rodriguez-Antolin A, Gomez-Veiga F, Alvarez-Osorio JK, Carballido-Rodriguez J, Palou-Redorta J, Solsona-Narbon E, Sanchez-Sanchez E, Unda M. Factors that predict the development of bone metastases due to prostate cancer: recommendations for follow-up and therapeutic options. Actas Urol Esp. 2014;38(4):263–9.
Rove KO, Crawford ED. Evolution of treatment options for patients with CRPC and bone metastases: bone-targeted agents that go beyond palliation of symptoms to improve overall survival. Oncology (Williston Park). 2011;25(14):1362–70 .1375-1381, 1387
Konishi H, Fujiya M, Ueno N, Moriichi K, Sasajima J, Ikuta K, Tanabe H, Tanaka H, Kohgo Y. MicroRNA-26a and -584 inhibit the colorectal cancer progression through inhibition of the binding of hnRNP A1-CDK6 mRNA. Biochem Biophys Res Commun. 2015;467(4):847–52.
Zhang X, Cheng SL, Bian K, Wang L, Zhang X, Yan B, Jia LT, Zhao J, Gammoh N, Yang AG, et al. MicroRNA-26a promotes anoikis in human hepatocellular carcinoma cells by targeting alpha5 integrin. Oncotarget. 2015;6(4):2277–89.
Ma DN, Chai ZT, Zhu XD, Zhang N, Zhan DH, Ye BG, Wang CH, Qin CD, Zhao YM, Zhu WP, et al. MicroRNA-26a suppresses epithelial-mesenchymal transition in human hepatocellular carcinoma by repressing enhancer of zeste homolog 2. J Hematol Oncol. 2016;9(1):1.
Erdmann K, Kaulke K, Thomae C, Huebner D, Sergon M, Froehner M, Wirth MP, Fuessel S. Elevated expression of prostate cancer-associated genes is linked to down-regulation of microRNAs. BMC Cancer. 2014;14:82.
Darshan M, Zheng Q, Fedor HL, Wyhs N, Yegnasubramanian S, Lee P, Melamed J, Netto GJ, Trock BJ, De Marzo AM, et al. Biobanking of derivatives from radical retropubic and robot-assisted laparoscopic prostatectomy tissues as part of the prostate cancer biorepository network. Prostate. 2014;74(1):61–9.
Song H, Liu Y, Pan J, Zhao ST. Expression profile analysis reveals putative prostate cancer-related microRNAs. Genet Mol Res. 2013;12(4):4934–43.
Mahn R, Heukamp LC, Rogenhofer S, von Ruecker A, Muller SC, Ellinger J. Circulating microRNAs (miRNA) in serum of patients with prostate cancer. Urology. 2011;77(5):1265 .e1269-1216
Borno ST, Fischer A, Kerick M, Falth M, Laible M, Brase JC, Kuner R, Dahl A, Grimm C, Sayanjali B, et al. Genome-wide DNA methylation events in TMPRSS2-ERG fusion-negative prostate cancers implicate an EZH2-dependent mechanism with miR-26a hypermethylation. Cancer Discov. 2012;2(11):1024–35.
Sandhu R, Rivenbark AG, Mackler RM, Livasy CA, Coleman WB. Dysregulation of microRNA expression drives aberrant DNA hypermethylation in basal-like breast cancer. Int J Oncol. 2014;44(2):563–72.
Sandhu R, Rivenbark AG, Coleman WB. Loss of post-transcriptional regulation of DNMT3b by microRNAs: a possible molecular mechanism for the hypermethylation defect observed in a subset of breast cancer cell lines. Int J Oncol. 2012;41(2):721–32.
Zhou C, Lu Y, Li X. miR-339-3p inhibits proliferation and metastasis of colorectal cancer. Oncol Lett. 2015;10(5):2842–8.
Guo L, Yu J, Yu H, Zhao Y, Chen S, Xu C, Chen F. Evolutionary and expression analysis of miR-#-5p and miR-#-3p at the miRNAs/isomiRs levels. BioMed Res Int. 2015;2015:168358.
Guo L, Zhang H, Zhao Y, Yang S, Chen F. Selected isomiR expression profiles via arm switching? Gene. 2014;533(1):149–55.
Koensgen D, Mustea A, Klaman I, Sun P, Zafrakas M, Lichtenegger W, Denkert C, Dahl E, Sehouli J. Expression analysis and RNA localization of PAI-RBP1 (SERBP1) in epithelial ovarian cancer: association with tumor progression. Gynecol Oncol. 2007;107(2):266–73.
Serce NB, Boesl A, Klaman I, von Serenyi S, Noetzel E, Press MF, Dimmler A, Hartmann A, Sehouli J, Knuechel R, et al. Overexpression of SERBP1 (plasminogen activator inhibitor 1 RNA binding protein) in human breast cancer is correlated with favourable prognosis. BMC Cancer. 2012;12:597.
Costa FC, Saito A, Goncalves KA, Vidigal PM, Meirelles GV, Bressan GC, Kobarg J. Ki-1/57 and CGI-55 ectopic expression impact cellular pathways involved in proliferation and stress response regulation. Biochim Biophys Acta. 2014;1843(12):2944–56.
Mayer B, Muche R. Formal sample size calculation and its limited validity in animal studies of medical basic research. Tierarztliche Praxis Ausgabe K, Kleintiere/Heimtiere. 2013;41(6):367–74.
Baker M. Reproducibility crisis: blame it on the antibodies. Nature. 2015;521(7552):274–6.
Mari Y, West GM, Scharager-Tapia C, Pascal BD, Garcia-Ordonez RD, Griffin PR. SERBP1 is a component of the liver receptor homologue-1 transcriptional complex. J Proteome Res. 2015;14(11):4571–80.
Acknowledgments
The study was supported by the Guangdong Natural Science Foundation (No. S201301004644) and the Southern Medical University Supporting Foundation (No. PX2015N015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Electronic supplementary material
Supplementary table 1
Listed were the siRNA sequences involved against SERBP1. (DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Guo, K., Zheng, S., Xu, Y. et al. Loss of miR-26a-5p promotes proliferation, migration, and invasion in prostate cancer through negatively regulating SERBP1. Tumor Biol. 37, 12843–12854 (2016). https://doi.org/10.1007/s13277-016-5158-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5158-z