Abstract
Many cell types release extracellular vesicles (EVs), including exosomes, microvesicles (MVs), and apoptotic bodies, which play a role in physiology and diseases. Presence and phenotype of circulating EVs in hematological malignancies (HMs) remain largely unexplored.
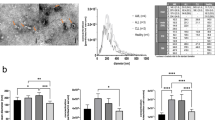
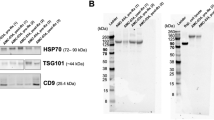
The aim of this study was to characterize EVs in peripheral blood of HM patients compared to healthy subjects (controls). We isolated serum EVs from patients with chronic lymphocytic leukemia (CLL), non-Hodgkin’s lymphoma (NHL), Waldenstrom’s macroglobulinemia (WM), Hodgkin’s lymphoma (HL), multiple myeloma (MM), acute myeloid leukemia (AML), myeloproliferative neoplasms (MPNs), myelodysplastic syndromes (MDS), and controls. EVs were isolated from serum of peripheral blood by ultracentrifuge steps and analyzed by flow cytometry to define count, size, and immunophenotype. MV levels were significantly elevated in WM, HL, MM, AML, and some MPNs and, though at a lesser degree, in CLL and NHL as compared to healthy controls. HL, MM, and MPNs generated a population of MVs characterized by lower size (below 0.3 μm) when compared to controls. MVs from patients specifically expressed tumor-related antigens, such as CD19 in B cell neoplasms, CD38 in MM, CD13 in myeloid tumors, and CD30 in HL. Both total and antigen-specific count of MVs significantly correlated with different HM clinical features such as Rai stage in CLL, International Prognostic Scoring System in WM, International Staging System in MM, and clinical stage in HL. MVs may represent a novel biomarker in HMs.








Similar content being viewed by others
References
Vader P, Breakefield XO, Wood MJA. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014;20(7):385–93.
El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–57.
Zocco D, Ferruzzi P, Cappello F, Kuo WP. Extracellular vesicles as shuttles of tumor biomarkers and anti-tumor drugs. Front Immunol. 2014;4:1–7.
Julich H, Willms A, Lukacs-Kornek V. Extracellular vesicle profiling and their use as potential disease specific biomarker. Front Immunol. 2014;5:1–7.
Revenfeld ALS, Bæk R, Nielsen MH, Stensballe A, Varming K, Jørgensen M. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin Ther. 2014;36(6):830–46.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–89.
Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–56.
Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26(5):1474–83.
Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72.
Del Conde I. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–11.
Lee Y, EL Andaloussi S, Wood MJA. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125–34.
Choi D-S, Kim D-K, Kim Y-K, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13(10–11):1554–71.
Thakur BK, Zhang H, Becker A, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Nat Publ Group. 2014;24(6):766–9.
Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208.
Caby MP. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–87.
Grant R, Ansa-Addo E, Stratton D, et al. A filtration-based protocol to isolate human plasma membrane-derived vesicles and exosomes from blood plasma. J Immunol Methods. 2011;371(1–2):143–51.
van der Meel R, Krawczyk-Durka M, van Solinge WW, Schiffelers RM. Toward routine detection of extracellular vesicles in clinical samples. Int J Lab Hematol. 2014;36(3):244–53.
Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracellular Vesicles. 2013;2:18389.
Dinkla S, Brock R, Joosten I, Bosman GJCGM. Gateway to understanding microparticles: standardized isolation and identification of plasma membrane-derived vesicles. Nanomedicine (Lond). 2013;8(10):1657–68.
Shet AS. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102(7):2678–83.
Jayachandran M, Litwiller RD, Owen WG, et al. Characterization of blood borne microparticles as markers of premature coronary calcification in newly menopausal women. Am J Physiol Heart Circ Physiol. 2008;295(3):H931–8.
George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982;60(4):834–40.
van der Vlist EJ, Nolte-‘t Hoen ENM, Stoorvogel W, Arkesteijn GJA, Wauben MHM. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat Protoc. 2012;7(7):1311–26.
Lacroix R, Robert S, Poncelet P, et al. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC collaborative workshop. J Thromb Haemost. 2010;8(11):2571–4.
Lacroix R, Judicone C, Poncelet P, et al. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10(3):437–46.
Van Der Pol E, Van Gemert M, Sturk A, Nieuwland R, Van Leeuwen T. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J Thromb Haemost. 2012;10(5):919–30.
Connor DE, Exner T, Ma DDF, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103(5):1044–52.
Rooney IA, Atkinson JP, Krul ES, et al. Physiologic relevance of the membrane attack complex inhibitory protein CD59 in human seminal plasma: CD59 is present on extracellular organelles (prostasomes), binds cell membranes, and inhibits complement-mediated lysis. J Exp Med. 1993;177(5):1409–20.
Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen‐presenting cell exosomes are protected from complement‐mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003;33(2):522–31.
Gyorgy B, Modos K, Pallinger E, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117(4):e39–48.
György B, Pálóczi K, Kovács A, et al. Improved circulating microparticle analysis in acid-citrate dextrose (ACD) anticoagulant tube. Thromb Res. 2014;133(2):285–92.
Fleitas T, Martínez-Sales V, Vila V, et al. Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS ONE. 2012;7(10):e47365.
Nozaki T, Sugiyama S, Sugamura K, et al. Prognostic value of endothelial microparticles in patients with heart failure. Eur J Heart Fail. 2014;12(11):1223–8.
Schmelzle M, Splith K, Andersen LW, et al. Increased plasma levels of microparticles expressing CD39 and CD133 in acute liver injury. Transplant J. 2013;95(1):63–9.
Sinning JM, Losch J, Walenta K, Bohm M, Nickenig G, Werner N. Circulating CD31+/annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J. 2011;32(16):2034–41.
Dragovic RA, Southcombe JH, Tannetta DS, Redman CWG, Sargent IL. Multicolor flow cytometry and nanoparticle tracking analysis of extracellular vesicles in the plasma of normal pregnant and pre-eclamptic women. Biol Reprod. 2013;89(6):151.
Van Aalderen M, Trappenburg M, Van Schilfgaarde M, et al. Procoagulant myeloblast-derived microparticles in AML patients: changes in numbers and thrombin generation potential during chemotherapy. J Thromb Haemost. 2011;9(1):223–6.
Ayers L, Kohler M, Harrison P, et al. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb Res. 2011;127(4):370–7.
Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115(9):1755–64.
Auwerda JJA, Yuana Y, Osanto S, et al. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb Haemost. 2011;105(1):14–20.
Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-1. Haematologica. 2011;96(9):1302–9.
Dey-Hazra E, Hertel B, Kirsch T, et al. Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. VHRM. 2010;6:1125–33.
Jy W, Horstman LL, Jimenez JJ, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2(10):1842–51.
Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109(4):175–80.
Abid Hussein MN, Meesters EW, Osmanovic N, Romijn FPHTM, Nieuwland R, Sturk A. Antigenic characterization of endothelial cell‐derived microparticles and their detection ex vivo. J Thromb Haemost. 2003;1(11):2434–43.
Berardi S, Caivano A, Ria R, et al. Four proteins governing overangiogenic endothelial cell phenotype in patients with multiple myeloma are plausible therapeutic targets. Oncogene. 2012;31(18):2258–69.
Terpos E, Tasidou A, Kastritis E, et al. Angiogenesis in waldenstrãm's macroglobulinemia. Clin Lymphoma Myeloma. 2011;9(1):46–9.
Marinaccio C, Nico B, Maiorano E, Specchia G, Ribatti D. Insights in hodgkin lymphoma angiogenesis. Leuk Res. 2014;38(8):857–61.
Cooper LJN, Al-Kadhimi Z, DiGiusto D, et al. Development and application of CD19-specific T cells for adoptive immunotherapy of B cell malignancies. Blood Cell Mol Dis. 2004;33(1):83–9.
Konoplev S, Medeiros LJ, Bueso-Ramos CE, Jorgensen JL, Lin P. Immunophenotypic profile of lymphoplasmacytic lymphoma/waldenström macroglobulinemia. Am J Clin Pathol. 2005;124(3):414–20.
Paiva B, Montes MC, a-Sanz RGI, et al. Multiparameter flow cytometry for the identification of the waldenstrom's clone in IgM-MGUS and waldenström macroglobulinemia: new criteria for differential diagnosis and risk stratification. Leukemia. 2013;28(1):166–73.
Domnikova NP, Dolgikh TY, Sholenberg EV, et al. Blood microvesicles during chronic lymphoproliferative diseases. Bull Exp Biol Med. 2013;156(1):94–7.
Nijhof IS, Groen RWJ, Noort WA, et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin Cancer Res. 2015;21(12):2802–10.
Arendt BK, Walters DK, Wu X, Tschumper RC, Jelinek DF. Multiple myeloma dell-derived microvesicles are enriched in CD147 expression and enhance tumor cell proliferation. Oncotarget. 2014;5(14):5686–99.
Küppers R, Engert A, Hansmann M-L. Hodgkin lymphoma. J Clin Invest. 2012;122(10):3439–47.
Hansen HP, Engels H-M, Dams M, et al. Protrusion-guided extracellular vesicles mediate CD30 trans-signalling in the microenvironment of Hodgkin's lymphoma. J Pathol. 2014;232(4):405–14.
Nadali G, Tavecchia L, Zanolin E, et al. Serum level of the soluble form of the CD30 molecule identifies patients with Hodgkin’s disease at high risk of unfavorable outcome. Blood. 1998;91(8):3011–6.
Blanc V, Bousseau A, Caron A, Carrez C, Lutz RJ, Lambert JM. SAR3419: An anti-CD19-maytansinoid immunoconjugate for the treatment of B-cell malignancies. Clin Cancer Res. 2011;17(20):6448–58.
Dahle J, Repetto-Llamazares AHV, Mollatt CS, et al. Evaluating antigen targeting and anti-tumor activity of a new anti-CD37 radioimmunoconjugate against non-Hodgkin’s lymphoma. Anticancer Res. 2013;33(1):85–95.
Oksvold MP, Kullmann A, Forfang L, et al. Expression of B-Cell surface antigens in subpopulations of exosomes released from B-Cell lymphoma cells. Clin Ther. 2014;36(6):847–862.e1.
Acknowledgments
The study was partially supported by Current Research Funds, Italian Ministry of Health.
No writing assistance was utilized in the production of this manuscript.
Compliance with ethical standards
During the entire investigation period, we followed guidelines and regulations of the Helsinki Declaration, and experiments were approved by the Ethics Committee of IRCCS-CROB (Prot 3 725; 7-2-2008). All patients and controls signed an informed consent form.
Conflicts of interest
No conflicts of interest were declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pellegrino Musto and Luigi Del Vecchio contributed equally to this work.
Rights and permissions
About this article
Cite this article
Caivano, A., Laurenzana, I., De Luca, L. et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumor Biol. 36, 9739–9752 (2015). https://doi.org/10.1007/s13277-015-3741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3741-3