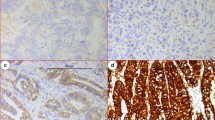
Abstract
Nuclear ubiquitous casein and cyclin-dependent kinases substrate (NUCKS) has been recently documented in various malignancies. However, data regarding the expression and prognostic value of NUCKS in gastric adenocarcinoma are limited. Specimens from 159 gastric adenocarcinoma patients who underwent primary gastrectomy were collected. Immunohistochemical method was used to evaluate NUCKS and Ki-67 expression. The correlations between NUCKS and clinical significance were analyzed. Elevated NUCKS expression was significantly associated with TNM stage (P = 0.034), depth of invasion (P = 0.001), and expression of Ki-67 (P = 0.035). Kaplan–Meier analysis indicated that NUCKS overexpression alone (P = 0.045 for overall survival) or in combination with Ki-67 (P = 0.007 for disease-free survival, P = 0.002 for overall survival) was correlated with adverse prognosis of the patients. Multivariate analysis revealed that combined NUCKS and Ki-67 overexpression was an independent prognostic factor for both disease-free survival (hazard ratio = 1.623, P = 0.02) and overall survival (hazard ratio = 1.667, P = 0.016) in gastric adenocarcinoma patients. The combination of NUCKS and Ki-67 overexpression in gastric adenocarcinoma further distinguished a subgroup of patients with poor prognosis.


Similar content being viewed by others
Reference
Ostvold AC, Holtlund J, Laland SG. A novel, highly phosphorylated protein, of the high-mobility group type, present in a variety of proliferating and non-proliferating mammalian cells. Eur J Biochem. 1985;153(3):469–75.
Ostvold AC, Norum JH, Mathiesen S, et al. Molecular cloning of a mammalian nuclear phosphoprotein NUCKS, which serves as a substrate for Cdk1 in vivo. Eur J Biochem. 2001;268(8):2430–40.
Bustin M, Reeves R. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog Nucleic Acid Res Mol Biol. 1996;54:35–100.
Grundt K, Skjeldal L, Anthonsen HW, et al. A putative DNA-binding domain in the NUCKS protein. Arch Biochem Biophys. 2002;407(2):168–75.
Wiśniewski JR, Zougman A, Krüger S, et al. Constitutive and dynamic phosphorylation and acetylation sites on NUCKS, a hypermodified nuclear protein, studied by quantitative proteomics. Proteins. 2008;73(3):710–8.
Walaas SI, Ostvold AC, Laland SG. Phosphorylation of P1, a high mobility group-like protein, catalyzed by casein kinase II, protein kinase C, cyclic AMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase II. FEBS Lett. 1989;258(1):106–8.
Anderson CW. DNA damage and the DNA-activated protein kinase. Trends Biochem Sci. 1993;18(11):433–7.
Meijer L, Ostvold AC, Walass SI, et al. High-mobility-group proteins P1, I and Y as substrates of the M-phase-specific p34cdc2/cyclincdc13 kinase. Eur J Biochem. 1991;196(3):557–67.
Azzi L, Meijer L, Ostvold AC, et al. Purification of a 15-kDa cdk4- and cdk5-binding protein. J Biol Chem. 1994;269(18):13279–88.
Sargent LM, Ensell MX, Ostvold AC, et al. Chromosomal changes in high-and low-invasive mouse lung adenocarcinoma cell strains derived from early passage mouse lung adenocarcinoma cell strains. Toxicol Appl Pharmacol. 2008;233(1):81–91.
Drosos Y, Kouloukoussa M, Østvold AC, et al. NUCKS overexpression in breast cancer. Cancer Cell Int. 2009;9:19.
Schaner ME, Ross DT, Ciaravino G, et al. Gene expression patterns in ovarian carcinomas. Mol Biol Cell. 2003;14(11):4376–86.
Kikuchi A, Ishikawa T, Mogushi K, et al. Identification of NUCKS1 as a colorectal cancer prognostic marker through integrated expression and copy number analysis. Int J Cancer. 2013;132(10):2295–302.
Naylor TL, Greshock J, Wang Y, et al. High resolution genomic analysis of sporadic breast cancer using array-based comparative genomichybridization. Breast Cancer Res. 2005;7(6):R1186–98.
Thompson HG, Harris JW, Wold BJ, et al. Identification and confirmation of a module of coexpressed genes. Genome Res. 2002;12(10):1517–22.
Grundt K, Haga IV, Aleporou-Marinou V, et al. Characterisation of the NUCKS gene on human chromosome 1q32.1 and the presence of a homologous gene in different species. Biochem Biophys Res Commun. 2004;323(3):796–801.
Jemal A, Bray F, Center MM, et al. Global Cancer Statistics. CA Cancer J Clin. 2011;61(2):69–90.
Gerdes J, Lemke H, Baisch H, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–5.
Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–22.
Inwald EC, Klinkhammer-Schalke M, Hofstädter F, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139(2):539–52.
King KL, Hwang JJ, Chau GY, et al. Ki-67 expression as a prognostic marker in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 1998;13(3):273–9.
Oshima CT, Iriya K, Forones NM. Ki-67 as a prognostic marker in colorectal cancer but not in gastric cancer. Neoplasma. 2005;52(5):420–4.
Mita S, Nakai A, Maeda S, et al. Prognostic significance of Ki-67 antigen immunostaining (MIB-1 monoclonal antibody) in ovarian cancer. J Nippon Med Sch. 2004;71(6):384–91.
Lee HE, Kim MA, Lee BL, et al. Low Ki-67 proliferation index is an indicator of poor prognosis in gastric cancer. J Surg Oncol. 2010;102(3):201–6.
Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC) cancer staging manual. 7th ed. Chicago: Springer; 2010.
Laurén P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49.
Cohen T, Prus D, Shia J, et al. Expression of P53, P27 and KI-67 in colorectal cancer patients of various ethnic origins: clinical and tissue microarray based analysis. J Surg Oncol. 2008;97(5):416–22.
Ajani JA, Barthel JS, Bekaii-Saab T, et al. Gastric cancer. Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer. Network. 2010;8(4):378–409.
Ziółkowski P, Gamian E, Osiecka B, et al. Immunohistochemical and proteomic evaluation of nuclear ubiquitous casein and cyclin-dependent kinases substrate in invasive ductal carcinoma of the breast. J Biomed Biotechnol. 2009;2009:919645.
Kovalenko OV, Golub EI, Bray-Ward P, et al. A novel nucleic acid-binding protein that interacts with human rad51 recombinase. Nucleic Acids Res. 1997;25(24):4946–53.
de Manzoni G, Verlato G, Tomezzoli A, et al. Study on Ki-67 immunoreactivity as a prognostic indicator in patients with advanced gastric cancer. Jpn J Clin Oncol. 1998;28(9):534–7.
Shitara K, Yatabe Y, Matsuo K, et al. Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer. 2013;16(2):261–7.
Victorzon M, Roberts PJ, Haglund C, et al. Ki-67 immunoreactivity, ploidy and S-phase fraction as prognostic factors in patients with gastric carcinoma. Oncology. 1996;53(3):182–91.
Kikuyama S, Kubota T, Shimizu K, et al. Ki-67 antigen expression in relation to clinicopathological variables and prognosis in gastric cancer. Oncol Rep. 1998;5(4):867–70.
Lazăr D, Tăban S, Sporea I, et al. Ki-67 expression in gastric cancer. Results from a prospective study with long-term follow-up. Rom J Morphol Embryol. 2010;51(4):655–61.
Bani-Hani KE, Almasri NM, Khader YS, Sheyab FM, et al. Combined evaluation of expressions of cyclin E and p53 proteins as prognostic factors for patients with gastric cancer. Clin Cancer Res. 2005;11(4):1447–53.
Li YZ, Zhao P. Expressions of cyclinB1, FHIT and Ki-67 in 336 gastric carcinoma patients and their clinicopathologic significance. Zhonghua Yi Xue Za Zhi. 2009;89(33):2337–41.
Tzanakis NE, Peros G, Karakitsos P, et al. Prognostic significance of p53 and Ki67 proteins expression in Greek gastric cancer patients. Acta Chir Belg. 2009;109(5):606–11.
Tsamandas AC, Kardamakis D, Tsiamalos P, et al. The potential role of Bcl-2 expression, apoptosis and cell proliferation (Ki-67 expression) in cases of gastric carcinoma and correlation with classic prognostic factors and patient outcome. Anticancer Res. 2009;29(2):703–9.
Acknowledgments
The authors are very grateful to Hongfei Ji for his technical assistance in the IHC. This work is supported by the National Natural Science Foundation of China (No. 81172265) and funds of The Affiliated Tumour Hospital of Harbin Medical University (No. JJZ2011-07).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, M., Wang, X., Zhao, Q. et al. Combined evaluation of the expression of NUCKS and Ki-67 proteins as independent prognostic factors for patients with gastric adenocarcinoma. Tumor Biol. 35, 7505–7512 (2014). https://doi.org/10.1007/s13277-014-1880-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1880-6