Abstract
Background
The biological clock allows an organism to anticipate periodic environmental changes and adjust its physiology and behavior accordingly.
Objective
This retrospective cross-sectional study examined circadian gene polymorphisms and clinical characteristics associated with insulin resistance (IR).
Methods
We analyzed data from 1,404 Korean adults aged 30 to 55 with no history of cancer and cardio-cerebrovascular disease. The population was classified according to sex and homeostasis model assessment of insulin resistance (HOMA-IR) values. Demographics, anthropometric and clinical characteristics, and single nucleotide polymorphisms (SNPs) were analyzed with respect to sex, age, and HOMA-IR values. We used association rule mining to identify sets of SNPs from circadian and metabolic sensing genes that may be associated with IR.
Results
Among the subjects, 15.0% of 960 women and 24.3% of 444 men had HOMA-IR values above 2. Most of the parameters differed significantly between men and women, as well as between the groups with high and low insulin sensitivity. Body fat mass of the trunk, which was significantly higher in insulin-resistant groups, had a higher correlation with high sensitivity C-reactive protein and hemoglobin levels in women, and alanine aminotransferase and aspartate aminotransferase levels in men. Homozygous minor allele genotype sets of SNPs rs17031578 and rs228669 in the PER3 gene could be more frequently found among women with HOMA-IR values above 2 (p = .014).
Conclusion
Oxidative stress enhanced by adiposity and iron overload, which may also be linked to NRF2 and PER3-related pathways, is related to IR in adulthood. However, due to the small population size in this study, more research is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The circadian rhythm, which is generated by endogenous oscillators, forms the temporal structure of human physiology by regulating energy metabolism in accordance with the natural light and dark cycle. When the circadian rhythm, gut microbial activities, and geophysical environmental signals are out of sync, energy metabolism can be disrupted, and conditions like insulin resistance may develop (Stenvers et al. 2019). Insulin sensitivity in skeletal muscle is remotely modulated by light and Sirtuin 1 (SIRT1) dependent pathway (Aras et al. 2019). It has been reported that certain polymorphic variants of SIRT1, as well as other circadian genes such as Circadian Locomotor Output Cycles Kaput (CLOCK), Brain and Muscle Arnt-Like 1 (BMAL1, also known as aryl hydrocarbon receptor nuclear translocator-like 1, ARNTL1), Cryptochrome 1 (CRY1) may lead to insulin resistance and increase the risk of metabolic and mood disorders (Dong et al. 2011; Dashti et al. 2014; Kovanen et al. 2015; Angelousi et al. 2018).
Various genome-wide association studies (GWASs) and meta-analyses of GWASs have identified single nucleotide polymorphisms (SNPs) from various loci associated with insulin resistance and type 2 diabetes (Hong et al. 2014). However, identifying the genes associated with disease phenotypes in complex, multifactorial disorders can be more challenging compared to that in simple Mendelian diseases. It is generally thought that the combined effects of multiple genetic variants with small effect sizes may constitute such complex traits. However, the genome-wide significance threshold at the level of p < 5.0 × 10− 8, which is applied in GWAS to prevent type 1 error, might filter SNPs with small effect sizes unless the sample size is extremely large. Therefore, additional methodologies might be necessary in order to compensate for the limitations of traditional GWASs (Stringer et al. 2011).
Breuer et al. (2018) addressed such issues of concern for GWAS of complex traits and hypothesized that analyzing the joint effect of genetic variants via the association rule mining (ARM) technique could be an alternative solution. ARM technique is a data mining technique that aims to extract frequent patterns from large databases. The technique is generally applied to analyze customer habits from transaction databases, and gene expression from microarray data. Breuer et al. considered this technique can unravel unknown associations between genome-wide genetic variants. Besides the genetic variation of specific SNPs, minor allele content (MAC) can be calculated as a quantitative measurement. Genome-wide accumulation of MAC in individuals might play a role in the pathogenesis of complex diseases such as type 2 diabetes and Alzheimer’s disease (Lei and Huang 2017; Chen et al. 2020). According to Chen et al. (2014) and Kido et al. (2018), it can be hypothesized that as the result of a natural selection process, risk alleles, compared to protective alleles might face negative selection and be kept at lower proportions. Therefore, risk alleles are more likely to be found among minor alleles.
From the above literature, we have concluded that individuals who carry certain variants of circadian genes may be more vulnerable to metabolic disorders. For the circadian genes, considering the joint effects of multiple variants and MAC might further enhance the prediction power of genome-wide association models. In this study, we will examine sex differences in circadian gene polymorphisms, and demographic and clinical characteristics associated with insulin resistance. Our hypothesis is: carrying certain sets of homozygous minor alleles or more minor alleles in the circadian and metabolic sensing genes may be metabolically riskier in the long term thus contributing to the pathogenesis of insulin resistance.
Materials and methods
Study design and settings
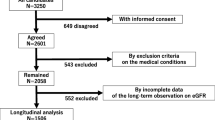
In this retrospective cross-sectional study, we analyzed the data of Korean adults (n = 1404) provided by the Korean medicine Data Center (KDC). The study protocol conformed to the declaration of Helsinki. Due to the retrospective nature of the work, this study was granted an exemption by the local ethics committee with a waiver of consent (Daejeon University institutional review board exemption: 1040647-202104-HR-019-03). The study population were relatively healthy adults recruited from the local community from 2017 to 2019, and had complete data on demographics, vital signs, anthropometric and bioelectric impedance measurements, hematological profile, and single nucleotide polymorphisms (SNPs). All data were anonymized, and the SNP data were analyzed on-site at the data center using a computer completely isolated from the internet and secured with a password and a locking device.
Study population
For this study, we used minimal inclusion and exclusion criteria so that the study population could be representative of the general population. The inclusion criterion was: relatively healthy adults recruited from the local community, who had complete data on demographics, vital signs, anthropometric and bioelectric impedance measurements, hematological profiles, and SNPs. The exclusion criterion was: those with a history of malignant tumor or cardio-cerebrovascular diseases.
Hematological profile
Overnight fasting blood samples (about 22.5 ml) were collected between 8 AM and 12 PM from the median cubital vein of each participant. The samples were centrifuged for 10 min at 3450 rpm and then transported to the laboratory (Seoul Clinical Laboratories, Yongin, Korea) within 24 h of collection for hematological examination. The blood test includes complete blood count, liver function, lipid profile, fasting plasma glucose and insulin levels, inflammatory markers, and differential count. Granulocyte count was calculated by summing the neutrophil, eosinophil and basophil counts. The granulocyte to lymphocyte ratio (GLR) was calculated by dividing the granulocyte count by the lymphocyte count, the neutrophil to lymphocyte ratio (NLR) was calculated by dividing the neutrophil count by the lymphocyte count, and the lymphocyte to monocyte ratio (LMR) was calculated by dividing the monocyte count by the lymphocyte count.
In this study, we used the homeostasis model assessment of insulin resistance (HOMA-IR), an indirect measurement of insulin resistance. HOMA-IR was calculated from fasting blood insulin and glucose levels. The primary cut-off point used for HOMA-IR in this study was 2, which corresponds to about the 75th percentile in the Korean population aged 40–69 years in Kim et al. (2018)’s study. The formula used for calculating HOMA-IR was as follows:
Measurements
Anthropometric measurements were obtained and recorded according to a standardized protocol. Height, weight, waist and hip circumferences were measured to the nearest ± 0.1 cm and kg. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters (kg/m2). Mass and proportion of body fat and muscle were measured with the InBody 770 bioelectrical impedance analyzer (Biospace, Seoul, Korea).
Gene analysis
SNP selection
A total of 750,050 SNPs were analyzed using the Theragen Precision Medicine Research Array (PMRA) chip (TheragenEtex Bio Institute, Suwon, Korea). Following genes that are involved in the circadian cycle and metabolic sensing (Rijo-Ferreira and Takahashi 2019; Stenvers et al. 2019) were selected for this study: AKT1, AKT2, ARNTL1, ARNTL2, CLOCK, CRY1, CRY2, DEC1, FOXO1, IRS1, JAK2, LIPE, LPL, MAPK8, NFE2L2, NFKB1, NPAS2, NR1D1, NR1D2, PBP4, PCK2, PER1, PER2, PER3, PNPLA2, RORA, RORB, RORC, SIRT1, SIRT6, SLC2A1, SLC2A2, SLC16A1, SLC16A4, SLC16A7, SREBF1, STAT3, STRA6, and TIMELESS. A list of SNPs for each gene was downloaded from the Single-Nucleotide Polymorphism database (National Center for Biotechnology Information (NCBI) dbSNP). Only common, intersecting SNPs from the selected genes and the Theragen PMRA chip were used for the analysis. All SNPs were autosomal SNPs. Before data analysis, SNPs were pruned using PLINK (v1.07) software (Purcell et al. 2007). SNPs with minor allele frequency and Hardy-Weinberg equilibrium of greater than 0.05 were selected. Then, SNPs with missingness per marker greater than 10%, and missingness per individual greater than 5%, were excluded. This resulted in 478 SNPs that were passed on to later processes.
Linkage disequilibrium (LD) in a specific population refers to a non-random association of alleles at different loci. LDs for the SNPs used in this study were calculated using PLINK software. In this study, we pruned SNPs with LD above the r2 threshold of 0.5 with PLINK option --indep-pairwise 20 3 0.5. This left us with 353 SNPs that were passed on to later processes. Further processes for selecting and pruning SNPs are shown in Fig. 1.
Exploratory association rule mining
For association rule mining, if the input data are too scarce, then rules can hardly be mined. Meanwhile, if all the SNPs including those with higher MAF are included in the association rule mining, then the homozygous minor allele genotypes with higher MAF will have a higher chance to be randomly associated with each other compared to those with lower MAF. Since risk alleles, in many cases, tend to be minor alleles with low MAF (Kido et al. 2018), we had to find a point of compromise by further pruning process. Therefore, from the SNPs used above, those with high MAF, in which homozygous minor allele genotype presented in more than 10% of the entire population were removed.
Genotype data of the 305 SNPs from 1404 individuals were obtained with PLINK software. The Association rule mining algorithm was programmed with Python 3.9.1 (Python Software Foundation, Wilmington, DE, USA) using the Mlxtend library (Raschka 2018). For association rule mining, minor allele-minor allele pairs were coded as 1 and minor allele-major allele pairs and major allele-major allele pairs were all coded as 0. All missing values in genotype data were assumed to be non-homozygous minor genotypes and thus were coded as 0. We created dummy variables and dropped the minor-major and major-major allele categories so that we could come up with association rules between homozygous minor allele genotypes as a result.
We divided the SNP dataset according to males and females with high and normal HOMA-IR values. From the entire dataset of n items, we can generate \(\sum _{k=2}^{n}\left(\genfrac{}{}{0pt}{}{n}{k}\right)\times ({2}^{k}-2)\) association rules. We then applied the apriori algorithm to the dataset to obtain candidates for the later association rule mining step. We used minimum support of 0.05 which is how often the item is present in the whole dataset. For the association rules, we used a threshold of 0.1 for minimum confidence. For the rules consisting of SNP sets of A and B, the support S is defined as follows: \(S\left(A\to B\right)=P\left(A\cap B\right)=N(A\cap B)/n\), where P stands for the probability and N stands for the number of sets. The support is used to select the frequently occurring sets from the entire dataset. Meanwhile, the confidence C is defined as follows: \(C\left(A\to B\right)=P\left(B|A\right)=N(A\cap B)/N\left(A\right)\). The confidence, in this case, is the probability of SNP B appears given the SNP A appears. The association rule mining algorithm looks for sets with a high level of support and confidence in relation to their threshold.
Minor allele content
MAC of each individual was counted from the above dataset of n = 478 SNPs for circadian genes, and n = 133 SNPs from core clock genes including ARNTL1, ARNTL2, CLOCK, CRY1, CRY2, DEC1, NPAS2, PER1, PER2, and PER3. Furthermore, we pruned the SNPs using different MAFs of 0.1 or 0.3.
Statistical analysis
The Hardy-Weinberg equilibrium, missing data, MAF, and LD were processed and calculated using PLINK software. The rest of the statistical analysis was performed using SPSS 23.0 for Windows (International Business Machines Corporation, Armonk, NY, USA) and Python 3.9.1 (Python Software Foundation, Wilmington, DE, USA). We applied log-normalization before analysis when appropriate. Males and females were analyzed separately. Differences were considered significant at P < .05. Correlation coefficients were interpreted as being negligible (0.00-0.10), weak (0.10–0.39), moderate (0.40–0.69), and strong (0.70 to 1.00).
Results
Population characteristics
Table 1 contains the demographic and clinical characteristics of the study population. The age of the population ranged from 30 to 55 years. Among the 1404 subjects, 960 (68.4%) were women and 444 (31.6%) were men. Most of the clinical characteristics differed significantly between men and women. Therefore, we conducted statistical analyses on men and women separately. Furthermore, because the number of male subjects was lower than that of female subjects, the statistical power of males in this study was lower than that of females. The mean ages of men and women were also found to be significantly different. The actual difference in the mean age, however, was about 1.5 years, so we considered that it was clinically negligible.
The distribution of HOMA-IR in men and women in this study population is shown in Table 2. HOMA-IR of 2, which was used as a cut-off point in this study, was at the 82nd percentile in the entire population, 85th percentile in women, and 76th percentile in men. Among the study population, 144 (15.0%) women and 108 (24.3%) men had HOMA-IR values above 2. There were significant differences in most of the clinical characteristics between the groups with HOMA-IR values above and below 2 (Table 1).
The hematological characteristics of the study population are shown in Table 3. Most of the hematological characteristics differed significantly between men and women, and between the groups with high and low insulin sensitivity (i.e., HOMA-IR value below and above 2). Subjects with a HOMA-IR value of 2 or higher had higher levels of high-sensitivity C-reactive protein (hsCRP), neutrophils, lymphocytes, monocytes, red blood cell (RBC) count, hemoglobin, and hematocrit, and lower mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) in both men and women. In addition, women with HOMA-IR 2 or higher had higher GLR and NLR.
The body impedance measurements of the study population are shown in Table 4. Compared to men, women had a greater visceral fat area (VFA), percentage of body fat (PBF), body fat mass (BFM) of the upper and lower limbs, fat to lean mass ratio (FLMR), fat to skeletal muscle mass ratio (FSMMR), and lean fat to trunk fat ratio (LFTFR). Meanwhile, men had greater soft lean mass (SLM) and skeletal muscle mass (SMM). BFM of the trunk did not differ between men and women. Subjects with HOMA-IR of 2 or higher had higher VFA, PBF, BFM, FLMR, SLM, SMM, FSMMR, and LFTFR.
Figure 2 summarizes the contents in Tables 1 and 3, and 4. Men and Women in the study population demonstrated both similar and different pathophysiological features of insulin resistance. The shared pathophysiological features of insulin resistance in men and women were increased levels of hsCRP and BFM of the trunk. The features that were found significant only in insulin-resistant women were elevated levels of low-density lipoprotein cholesterol (LDL-C), GLR, NLR, and LFTFR. Meanwhile, BMI, blood pressure, fasting plasma insulin and glucose, liver enzymes, triglyceride, neutrophils, lymphocytes, monocytes, RBC count, hemoglobin, and hematocrit were all found to be significantly elevated in the insulin-resistant population and they were also significantly higher in men. VFA, PBF, FLMR, and FSMMR were significantly elevated in the insulin-resistant population and they were also significantly higher in women. Furthermore, correlations between levels of inflammatory markers, trunk fat mass, low-density lipoprotein cholesterol, liver enzymes, hemoglobin, and hematocrit in men and women are shown in Fig. S1. Correlations between HOMA-IR and clinical parameters in men and women in different age groups are shown in Table S13.
Shared and unique features of insulin resistance between men and women. LDL-C, low-density lipoprotein cholesterol; GLR, granulocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LFTFR, lean fat to trunk fat ratio; hsCRP, high sensitivity C-reactive protein; BMI, body mass index; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin, HDL-C, high-density lipoprotein cholesterol; VFA, visceral fat area; PBF, percent body fat; FLMR, fat to lean mass ratio; FSMMR, fat to smooth muscle mass ratio
Exploratory association rule mining
N = 283, 309, 143, 411 candidate rules were stratified for the group of (1) females and (2) males with high insulin sensitivity (normal HOMA-IR values), and (3) females and (4) males with low insulin sensitivity (high HOMA-IR values), respectively. The cut-off value of ≥ 2 was used to differentiate between normal and high HOMA-IR values. Supports ranged from 0.050 to 0.10 and confidence ranged from 0.46 to 1 in the outcome association rules.
Breuer et al. (2014) used binary variables in their work by coding homozygous minor alleles and heterozygous major-minor allele pairs as a single variable, and homozygous major alleles as another variable. Their resulting association rules consisted of sets of SNPs from different genes. However, in our study, we adopted a slightly different approach by coding homozygous minor alleles as one variable, and heterozygous minor-major allele pairs and homozygous major alleles into another variable, and dropped the latter variable when generating dummy variables. Therefore, in this study, we searched for the association patterns of homozygous minor alleles of the circadian and metabolic sensing genes. This resulted in the rules consisting of SNPs from the same genes only. It may be due to the relatively small sample of genes examined in this study, and linkage disequilibrium as proposed by Zondervan and Cardon (2004). Since some of the association rules were shared among all groups while other rules only appeared in certain groups, we compiled all the rules from groups with different clinical features altogether. Among the rules, those that were shared but displayed different frequencies between the groups (examined by the Chi-square test with a significance level of 0.10) are shown in Table 5. The characteristics of the SNPs in Table 5 are shown in Table 6. Considering the significance level of 0.05, only one set of SNPs in the PER3 gene was significant. However, the statistical power of this study was low and this significance would vanish if we consider the Bonferroni correction. Detailed results are attached as supplementary data, Tables S1–12.
Homozygous minor allele genotype sets of SNPs rs10930781, rs2364720, rs10188107, and rs4243387 (NFE2L2 gene) and rs17031578 and rs228669 (PER3 gene) could be more frequently found among females with HOMA-IR ≥ 2 (p = .072 for homozygous minor genotypes in SNP clusters of NFE2L2 and p = .014 for that of PER3) although the estimated odds ratio for those variations were not high (1.8 for homozygous minor genotypes in SNP clusters of NFE2L2 and 2.1 for that of PER3 in women). Those sets of SNPs could be more frequently found among males with HOMA-IR ≥ 2 as well, although the results were not statistically significant.
Effect of MAC in the pathophysiology of insulin resistance
MAC of specific genes for each individual was calculated as described in the methods and in Fig. 1. Pearson’s correlation analysis was performed between HOMA-IR and different MAC categories constructed using circadian genes and core clock genes as previously mentioned in the methods section. We performed the correlation analysis on groups of different ages and sex. Log-normalized HOMA-IR values and MAC categories did not show any correlation in female groups in their 30s, 40s, and 50s. Meanwhile, in males, different age groups showed different patterns of correlation between HOMA-IR values and MAC categories. For males in their 50s, homozygous minor/minor genotype count of core clock genes with MAF < 0.3 had a weak positive correlation (r = .2) with HOMA-IR values at the significance level of 0.05. For males in their 30 and 40 s, however, homozygous minor/minor genotype count of core clock genes with MAF < 0.3 was negatively correlated (r=-.12 without statistical significance, r=-.13 with marginal significance, respectively). The results are displayed in Table 7.
Discussion
Insulin, an anabolic peptide hormone, is produced, stored, and secreted by the pancreatic beta cells in response to a glycemic stimulus. In healthy adults, β-cell responsiveness and insulin sensitivity are both higher in the morning and lower in the evening as they are under circadian control (Saad et al. 2012). In addition, plasma glucose and hepatic gluconeogenesis are also regulated by circadian metabolic rhythm (Simcox et al. 2015). Insulin resistance is a complex phenomenon that can be caused by diverse pathways. Proteomics studies revealed that there are different pathological processes of insulin resistance in skeletal muscle, adipose tissue, and liver (Li et al. 2020). Men and women have different body composition and physiology of fat and muscle, and the difference may lead to sex differences in the pathophysiology of insulin resistance. In general, women rely more on lipid metabolism while men tend to prefer utilizing glucose in a situation where energy is needed. Exercising induces a greater degree of lipolysis in women compared to that in men. Meanwhile, the carbohydrate oxidation rate is higher in men compared to that in women (Hedrington and Davis 2015). It might be due to the fact that the skeletal muscle is a major site for glucose uptake and decomposition. Men on average, compared to women, have greater skeletal muscle mass. It is well known that greater skeletal muscle mass is associated with increased insulin sensitivity while sarcopenia is known to be related to insulin resistance (Moon 2013).
In addition to metabolic preferences, lipid metabolism itself displays sexual dimorphism. For instance, the aquaglyceroporin (AQP) expression pattern is different in males and females. AQPs are channel protein that facilitates glycerol transportation across the cell membrane. AQPs are generally expressed in adipocytes and hepatocytes. In rodents, males have higher hepatic AQP9 protein, and thus greater hepatic glycerol permeability. Similar tendencies are observed in humans as men have higher hepatic glycerol permeability compared to women (Rodríguez et al. 2015). In this study, serum levels of liver enzymes were significantly higher in men and in subjects with HOMA-IR values above 2. In addition, there were stronger correlations between BFM of the trunk and ALT and AST in men compared to those in women. It could be possible that in insulin-resistant men, hepatic cell damage and the resulting oxidative stress and inflammation could be more prominent. In a community-based study by Cho et al., an increase in liver enzyme activities was accompanied by an increase in C-reactive protein in diabetic patients, and both of them were independent predictors for type 2 diabetes (Cho et al. 2007). In men, the accumulation of fat in the liver, central obesity, and inflammation may be one of the common pathophysiologies of insulin resistance and type 2 diabetes. These results may partially explain the higher incidence of fatty liver disease in men as well (Rodríguez et al. 2015).
On the other hand, the accumulation of body fat is related to chronic inflammatory conditions (Fernández-Sánchez et al. 2011). In this study, women, on average, had a higher percentage of body fat and FLMR compared to those of men. Parameters such as LDL-C, GLR, and NLR were elevated and LFTFR was decreased in insulin-resistant women but not in insulin-resistant men. In addition, insulin-resistant women had a higher level of hsCRP in their plasma compared to insulin-resistant men. There were stronger correlations between hsCRP and NLR and BFM of the trunk in women compared to those in men (Fig. S1). From these results, it could be postulated that women are more prone to systemic inflammation and oxidative stress due to higher adiposity.
Besides adiposity, elevated RBC count, hemoglobin levels and hematocrit were all related to insulin resistance in this study population. Meanwhile, hsCRP was weakly correlated with Hb and Hct in women only. Many studies have suggested that insulin has an erythropoietic effect and insulin receptor is engaged in RBC proliferation. It was previously described by Choi et al. that increased erythropoiesis and chronic subclinical inflammation are related to insulin resistance in elderly Koreans (Choi et al. 2003). Iron is a major source of hydroxyl radical (•OH) which can cause a wide range of biological damage. Insulin resistance is associated with iron overload as insulin can stimulate ferritin synthesis and facilitate iron uptake by cells. It has been recently discovered that iron overload can predict early death in a dose-dependent manner in the general population (Fernández-Real et al. 2015). Prolonged iron overload can lead to decreased lysosomal pools, autophagy failure, glucose intolerance and insulin resistance. Interventions such as an iron restriction diet, blood-letting, and chelation therapy could all increase insulin sensitivity (Jahng et al. 2019).
From the exploratory association rule mining, we could see that sets consisting of homozygous minor allele genotypes of SNPs rs10930781, rs2364720, rs10188107, and rs4243387 (from NFE2L2 gene) and rs17031578 and rs228669 (from PER3 gene) could be more frequently found among females with HOMA-IR ≥ 2 although the estimated odds ratio for those variations (1.8 for homozygous minor genotypes in SNP clusters of NFE2L2 and 2.1 for that of PER3; all odds ratios may be applied to women only) are not that high. SNPs rs10930781, rs2364720, rs10188107, and rs4243387 are all intron variants of the NFE2L2 gene. They have been reported in the studies on acute respiratory distress syndrome and adult-onset cognitive deficits induced by Phencyclidine as possible functional variants in the NFE2L2 gene (Acosta-Herrera et al. 2015; Shirai et al. 2015) NFE2L2 (Nuclear factor-erythroid 2 like, also known as NRF2) is a transcription factor that regulates redox metabolism in cells. NFE2L2 activity increases during various stressful situations, including inflammation and redox perturbation to direct metabolic reprogramming of the cells and protect cells from oxidative damage. NFE2L2 is necessary for antioxidant function and nicotinamide adenine dinucleotide phosphate (NADPH) production in cells and tissues (Hayes and Dinkova-Kostova 2014; Uruno et al. 2015). In addition, NFE2L2 can regulate circadian rhythm and integrate redox metabolism into the tissue-specific circadian cycle by binding to enhancer regions of CRY2 and the stress response element (STRE) motif of PER3 and regulating their expression (Wible et al. 2018).
The cytoprotective and antioxidative activity of Kelch Like ECH Associated Protein 1 (KEAP1)-NRF2 system is important for glucose homeostasis as it can protect pancreatic beta cells from oxidative damage. Moreover, NRF2 plays an important role in iron metabolism. Iron can mediate glucose metabolism at multiple levels, and increased iron load can promote oxidative stress via the Fenton reaction (Kerins and Ooi 2018). NRF2 can restrict inflammation and protect cells from oxidative damage by regulating the pentose phosphate pathway (PPP). The PPP is also important for glutathione reduction, NADPH production, and nucleic acid biosynthesis (Early et al. 2018). Therefore, dysregulation of NRF2 may render cells vulnerable to oxidative stress and chronic inflammation. NRF2 can be a potential target for the treatment of chronic inflammation, insulin resistance, and type 2 diabetes (David et al. 2017). It has been recently discovered that Nrf2 can restore leptin and insulin sensitivity by reducing the oxidative stress produced by the hypothalamus in mice (Yagishita et al. 2017).
Meanwhile, rs228669 SNP in the PER3 gene is a synonymous variant while rs17031578 is an intron variant. In Kovac et al.’s study, SNP rs228669 was significantly associated with increased levels of triglycerides (TG) and risk of pre-term birth (Kovac et al. 2019). In Zhang et al.’s study, rs228669 SNP was significantly associated with the overall survival of patients with hepatocellular carcinoma. SNP rs228669 is located in the exonic splicing enhancer (ESE) region. Therefore, when there is a variation, it is possible that the sequence of mRNA, and structures and functions of the product protein can be altered (Zhang et al. 2014). Although it is not directly related to those SNPs above, PER3 length polymorphism in its exon 18 has been reported in patients with type 2 diabetes (Karthikeyan et al. 2014). In addition, the transcript level of PER3 was reduced in type 2 diabetes patients (Ando et al. 2009). It has been recently discovered that adipogenesis is regulated by the circadian rhythm generated by Per3 in adipocyte precursor cells (APCs), and deletion of the Per3 gene is related to increased adipogenesis in vivo in mice. In APCs in mice, the Per3-Bmal1 complex can regulate adipogenesis by modulating Klf15 (Kruppel-like factor 15) and Pparγ (peroxisome proliferator-activated receptor γ). Meanwhile, in human cells, there are conflicting results. Some studies reported that human PER3 repressed adipogenesis in human mesenchymal stem cells and downregulation of human PER3 immortalized bone marrow-derived Scp-1 cells and patient adipose-derived stromal cells. On the other hand, PER3 enhanced adipogenesis in human adipose tissue-derived stromal cells (Aggarwal et al. 2017; Wan et al. 2021). Further research may be necessary to clarify the relationship of the PER3 variations to the development of insulin resistance in humans.
Recently, Jakubiak et al. (2021) discovered that obesity and insulin resistance had the strongest relationship with oxidative stress among metabolic syndrome components such as “obesity and insulin resistance,” “dyslipidemia,” and “blood pressure.” It is worth noting that NRF2 and PER3 are related to the redox cycle and adipogenesis. According to Rey et al. (2016), inhibiting the PPP can alter circadian rhythm through mechanisms independent of the transcription-translation feedback loop and NRF2 is a key mediator linking the redox signals and circadian oscillations. 6-aminonicotinamide (6AN) treatment, which inhibits PPP and NADPH production, could delay the circadian phase in a reversible manner. Circadian genes, including PER3, displayed phase delays in transcription profiles as well. 6AN treatment also induced NRF2 activation and subsequent PER3 response. NRF2 has been shown to bind to the STRE site and mediate Per3 expression (Wang et al. 2012).
Meanwhile, the roles of NRF2 and PER3 in human adipogenesis still remain uncertain. Despite the fact that evidence from the majority of studies suggests that oxidative stress can upregulate Nrf2, which in turn promotes adipogenesis. Nrf2 inhibition can reduce lipid accumulation in response to oxidative stress. However, conflicting evidence suggests that Nrf2 can inhibit adipogenesis through the aromatic receptor pathway. More research is needed to clarify the pathway and fully comprehend the relationship between circadian rhythm, oxidative stress, obesity, and insulin resistance.
For the MAC of the circadian genes, it was found that homozygous minor genotype count of the circadian genes with MAF below < 0.3 were significantly correlated with HOMA-IR only in men in their 50s, although the correlation was very modest (r = .200, p < .05). For women, MAC or homozygous minor genotype count of the circadian genes did not display any significant correlation with HOMA-IR. It was interesting to note that homozygous minor/minor genotype count for the core clock genes with MAF < 0.03 was negatively correlated with HOMA-IR in men in their 30s (r=-.12) and 40s (r=-.13). However, for men in their 50s, the variables were positively correlated (r = .2). It might be due to aging-associated changes in gene expression. Further investigation is required. In a previous study conducted on the British population, it was found that enrichment of minor alleles of SNPs was found in patients with type 2 diabetes (Lei and Huang 2017). SNPs from genes other than circadian and metabolic sensing genes may need to be included in the model to enhance the predictive capability of MAC for insulin resistance and type 2 diabetes.
The goal of this study was to discover the minor alleles that might be related to the pathogenesis of insulin resistance. From this study, we have found that body composition and physiological differences in men and women may lead to sex differences in the pathophysiology of insulin resistance. Insulin-resistant women, compared to insulin-resistant men, had a greater degree of systemic inflammation and adiposity. Meanwhile, insulin-resistant men had greater levels of liver enzymes, plasma insulin and glucose, and hematological parameters such as hematocrit compared to those in insulin-resistant women.
From association rule mining results, we could find that homozygous minor allele contents in a circadian gene such as PER3 may predispose individuals to be more prone to insulin resistance although additional evidence from further research is necessary. In this study, homozygous minor alleles of PER3 could be more frequently found among women with decreased insulin sensitivity (p = .014). It may be due to the fact that women tend to rely more on lipid metabolism and alterations in the PER3 gene may affect lipid metabolism. Meanwhile, homozygous minor alleles of NFE2L2 could also be more frequently found among women with insulin resistance, although the result was not statistically significant (p = .072). The degree of systemic inflammation measured with hsCRP, GLR, and NLR was greater in insulin-resistant women compared to that in insulin-resistant men. Due to the higher level of systemic low-grade inflammation, women with decreased insulin sensitivity may be more prone to oxidative stress. However, because the number of female subjects was greater than that of male subjects, females had greater statistical power in this study than males. As a result, the greater significance observed in female groups could be a result of the power difference as well.
To conclude, oxidative stress due to adiposity and iron overload is related to insulin resistance in early to middle adulthood. Homozygous minor alleles of genes such as PER3 and MAC of the circadian genes may predispose individuals to insulin resistance. However, the population in this research may be relatively too young and small to draw a meaningful conclusion. Therefore, further investigation of a larger population is necessary.
Limitations
There are several limitations to this study. First, since the number of subjects was not large, our data have to be interpreted with careful consideration. We did not apply multiple comparison corrections for gene analysis because we only applied planned comparisons on specific SNPs. The results may no longer be significant if we apply Bonferroni correction. In this study, we primarily used the PLINK program for data processing and association rule mining for the main analysis. The reason for this is that the number of subjects was small and insufficient to perform GWAS. Rather than searching for SNPs associated with insulin resistance throughout the entire pool, the authors focused on the genes associated with the mechanism of insulin resistance and then looked for association rules within the SNPs of those genes. Searching for SNP sets rather than individual SNPs will also result in a larger effect size. Duplication studies with a larger population may be necessary to further verify and supplement the present investigation. In addition, there were fewer men compared to women in this study. So, the power of statistical tests may differ between men and women. The age of the population of this study is 30 to 55, which may be relatively young compared to other similar studies. Further research on the older population is necessary to apply the results to the population older than 55 years. Finally, we did not check the smoking status, drug history, and sleep and dietary patterns of the subjects in this study, which might have affected the health status of the participants. Further studies may be needed on those aspects as well.
Change history
07 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s13258-024-01493-y
References
Acosta-Herrera M, Pino-Yanes M, Blanco J, Ballesteros JC, Ambrós A, Corrales A, Gandía F, Subirá C, Domínguez D, Baluja A et al (2015) Common variants of NFE2L2 gene predisposes to acute respiratory distress syndrome in patients with severe sepsis. Crit Care 19:1–8. https://doi.org/10.1186/s13054-015-0981-y
Aggarwal A, Costa MJ, Rivero-Gutiérrez B, Ji L, Morgan SL, Feldman BJ (2017) The circadian clock regulates adipogenesis by a Per3 crosstalk pathway to Klf15. Cell Rep 21:2367–2375. https://doi.org/10.1016/j.celrep.2017.11.004
Ando H, Takamura T, Matsuzawa-Nagata N, Shima KR, Eto T, Misu H, Shiramoto M, Tsuru T, Irie S, Fujimura A et al (2009) Clock gene expression in peripheral leucocytes of patients with type 2 diabetes. Diabetologia 52:329–335. https://doi.org/10.1007/s00125-008-1194-6
Angelousi A, Kassi E, Nasiri-Ansari N, Weickert MO, Randeva H, Kaltsas G (2018) Clock genes alterations and endocrine disorders. Eur J Clin Investig 48:e12927. https://doi.org/10.1111/eci.12927
Aras E, Ramadori G, Kinouchi K, Liu Y, Ioris RM, Brenachot X, Ljubicic S, Veyrat-Durebex C, Mannucci S, Galie M et al (2019) Light entrains diurnal changes in insulin sensitivity of skeletal muscle via ventromedial hypothalamic neurons. Cell Rep 27:2385–2398. https://doi.org/10.1016/j.celrep.2019.04.093
Breuer R, Mattheisen M, Frank J, Krumm B, Treutlein J, Kassem L, Strohmaier J, Herms S, Mühleisen TW, Degenhardt F et al (2018) Detecting significant genotype–phenotype association rules in bipolar disorder: market research meets complex genetics. Int J Bipolar Disord 6:1–10. https://doi.org/10.1186/s40345-018-0132-x
Chan Y, Lim ET, Sandholm N, Wang SR, McKnight AJ, Ripke S, Daly MJ, Neale BM, Salem RM, Hirschhorn JN et al (2014) An excess of risk-increasing low-frequency variants can be a signal of polygenic inheritance in complex diseases. Am J Hum Genet 94:437–452. https://doi.org/10.1016/j.ajhg.2014.02.006
Chen H, Lei X, Yuan D, Huang S (2020) The relationship between the minor allele content and Alzheimer’s disease. Genomics 112:2426–2432. https://doi.org/10.1016/j.ygeno.2020.01.015
Cho NH, Jang HC, Choi SH, Kim HR, Lee HK, Chan JC, Lim S (2007) Abnormal liver function test predicts type 2 diabetes: a community-based prospective study. Diabetes Care 30:2566–2568. https://doi.org/10.2337/dc07-0106
Choi KM, Lee J, Kim YH, Kim KB, Kim DL, Kim SG, Shin DH, Kim NH, Park IB, Choi DS et al (2003) Relation between insulin resistance and hematological parameters in elderly Koreans—Southwest Seoul (SWS) Study. Diabetes Res Clin Pract 60:205–212. https://doi.org/10.1016/S0168-8227(03)00059-7
Dashti HS, Smith CE, Lee YC, Parnell LD, Lai CQ, Arnett DK, Ordovás JM, Garaulet M (2014) CRY1 circadian gene variant interacts with carbohydrate intake for insulin resistance in two independent populations: Mediterranean and North American. Chronobiol Int 31:660–667. https://doi.org/10.3109/07420528.2014.886587
David JA, Rifkin WJ, Rabbani PS, Ceradini DJ (2017) The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res 2017:4826724. https://doi.org/10.1155/2017/4826724
Dong Y, Guo T, Traurig M, Mason CC, Kobes S, Perez J, Knowler WC, Bogardus C, Hanson RL, Baier LJ (2011) SIRT1 is associated with a decrease in acute insulin secretion and a sex specific increase in risk for type 2 diabetes in Pima Indians. Mol Genet Metab Rep 104:661–665. https://doi.org/10.1016/j.ymgme.2011.08.001
Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, Palsson-McDermott EM, Angiari S, Ryan DG, Corcoran SE et al (2018) Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc Natl Acad Sci 115:E8460–E8468. https://doi.org/10.1073/pnas.1800431115
Fernández-Real JM, McClain D, Manco M (2015) Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care 38:2169–2176. https://doi.org/10.2337/dc14-3082
Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González Á, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA (2011) Inflammation, oxidative stress, and obesity. Int J Mol Sci 12:3117–3132. https://doi.org/10.3390/ijms12053117
Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39:199–218. https://doi.org/10.1016/j.tibs.2014.02.002
Hedrington MS, Davis SN (2015) Sexual dimorphism in glucose and lipid metabolism during fasting, hypoglycemia, and exercise. Front Endocrinol 6:61. https://doi.org/10.3389/fendo.2015.00061
Hong KW, Chung M, Cho SB (2014) Meta-analysis of genome-wide association study of homeostasis model assessment β cell function and insulin resistance in an east asian population and the european results. Mol Genet Genomic Med 289:1247–1255. https://doi.org/10.1007/s00438-014-0885-6
Jahng JW, Alsaadi RM, Palanivel R, Song E, Hipolito VE, Sung HK, Botelho RJ, Russell RC, Sweeney G (2019) Iron overload inhibits late stage autophagic flux leading to insulin resistance. EMBO Rep 20:e47911. https://doi.org/10.15252/embr.201947911
Jakubiak GK, Osadnik K, Lejawa M, Osadnik T, Goławski M, Lewandowski P, Pawlas N (2021) Obesity and insulin resistance is the component of the metabolic syndrome most strongly Associated with oxidative stress. Antioxidants 11:79. https://doi.org/10.3390/antiox11010079
Karthikeyan R, Marimuthu G, Sooriyakumar M, BaHammam AS, Spence DW, Pandi-Perumal SR, Brown GM, Cardinali DP (2014) Per3 length polymorphism in patients with type 2 diabetes mellitus. Horm Mol Biol Clin Investig 18:145–149. https://doi.org/10.1515/hmbci-2013-0049
Kerins MJ, Ooi A (2018) The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal 29:1756–1773. https://doi.org/10.1089/ars.2017.7176
Kido T, Sikora-Wohlfeld W, Kawashima M, Kikuchi S, Kamatani N, Patwardhan A, Chen R, Sirota M, Kodama K, Hadley D et al (2018) Are minor alleles more likely to be risk alleles? BMC Med Genom 11:1–11. https://doi.org/10.1186/s12920-018-0322-5
Kim B, Choi HY, Kim W, Ahn C, Lee J, Kim JG, Kim J, Shin H, Yu JM, Moon S (2018) The cut-off values of surrogate measures for insulin resistance in the korean population according to the Korean Genome and Epidemiology Study (KOGES). PLoS ONE 13:e0206994. https://doi.org/10.1371/journal.pone.0206994
Kovac U, Jasper EA, Smith CJ, Baer RJ, Bedell B, Donovan BM, Weathers N, Prosenc Zmrzljak U, Jelliffe-Pawlowski LL, Rozman D et al (2019) The association of polymorphisms in circadian clock and lipid metabolism genes with 2nd trimester lipid levels and preterm birth. Front Genet 10:540. https://doi.org/10.3389/fgene.2019.00540
Kovanen L, Donner K, Partonen T (2015) SIRT1 polymorphisms associate with seasonal weight variation, depressive disorders, and diastolic blood pressure in the general population. PLoS ONE 10:e0141001. https://doi.org/10.1371/journal.pone.0141001
Lei X, Huang S (2017) Enrichment of minor allele of SNPs and genetic prediction of type 2 diabetes risk in british population. PLoS ONE 12:e0187644. https://doi.org/10.1371/journal.pone.0187644
Li Y, Ma Q, Li P, Wang J, Wang M, Fan Y, Wang T, Wang C, Wang T, Zhao B (2020) Proteomics reveals different pathological processes of adipose tissue, liver, and skeletal muscle under insulin resistance. J Cell Physiol 235:6441–6461. https://doi.org/10.1002/jcp.29658
Moon SS (2013) Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr J EJ 13–0244. https://doi.org/10.1507/endocrj.EJ13-0244
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, De Bakker PI, Daly MJ et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–75. https://doi.org/10.1086/519795
Raschka S (2018) MLxtend: providing machine learning and data science utilities and extensions to Python’s scientific computing stack. J Open Source Softw 3:638. https://doi.org/10.21105/joss.00638
Rey G, Valekunja UK, Feeney KA, Wulund L, Milev NB, Stangherlin A, Ansel-Bollepalli L, Velagapudi V, O’Neill JS, Reddy AB (2016) The pentose phosphate pathway regulates the circadian clock. Cell Metab 24:462–473. https://doi.org/10.1016/j.cmet.2016.07.024
Rijo-Ferreira F, Takahashi JS (2019) Genomics of circadian rhythms in health and disease. Genome Med 11:1–6. https://doi.org/10.1186/s13073-019-0704-0
Rodríguez A, Marinelli RA, Tesse A, Frühbeck G, Calamita G (2015) Sexual dimorphism of adipose and hepatic aquaglyceroporins in health and metabolic disorders. Front Endocrinol 6:171. https://doi.org/10.3389/fendo.2015.00171
Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC et al (2012) Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61:2691–2700. https://doi.org/10.2337/db11-1478
Shirai Y, Fujita Y, Hashimoto R, Ohi K, Yamamori H, Yasuda Y, Ishima T, Suganuma H, Ushida Y, Takeda M et al (2015) Dietary intake of sulforaphane-rich broccoli sprout extracts during juvenile and adolescence can prevent phencyclidine-induced cognitive deficits at adulthood. PLoS ONE 10:e0127244. https://doi.org/10.1371/journal.pone.0127244
Simcox JA, Mitchell TC, Gao Y, Just SF, Cooksey R, Cox J, Ajioka R, Jones D, Lee SH, King D et al (2015) Dietary iron controls circadian hepatic glucose metabolism through heme synthesis. Diabetes 64:1108–1119. https://doi.org/10.2337/db14-0646
Stenvers DJ, Scheer FA, Schrauwen P, la Fleur SE, Kalsbeek A (2019) Circadian clocks and insulin resistance. Nat Rev Endocrinol 15:75–89. https://doi.org/10.1038/s41574-018-0122-1
Stringer S, Wray NR, Kahn RS, Derks EM (2011) Underestimated effect sizes in GWAS: fundamental limitations of single SNP analysis for dichotomous phenotypes. PLoS ONE 6:e27964. https://doi.org/10.1371/journal.pone.0027964
Uruno A, Yagishita Y, Yamamoto M (2015) The Keap1–Nrf2 system and diabetes mellitus. Arch Biochem Biophys 566:76–84. https://doi.org/10.1016/j.abb.2014.12.012
Wan X, Zhu L, Zhao L, Peng L, Xiong J, Yang W, Yuan J, Liang F, Zhang K, Chen K (2021) hPER3 promotes adipogenesis via hHSP90AA1-mediated inhibition of Notch1 pathway. Cell Death Dis 12:1–15. https://doi.org/10.1038/s41419-021-03584-0
Wang X, Mozhui K, Li Z, Mulligan MK, Ingels JF, Zhou X, Hori RT, Chen H, Cook MN, Williams RW et al (2012) A promoter polymorphism in the Per3 gene is associated with alcohol and stress response. Transl Psychiatry 2:e73. https://doi.org/10.1038/tp.2011.71
Wible RS, Ramanathan C, Sutter CH, Olesen KM, Kensler TW, Liu AC, Sutter TR (2018) NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife 7:e31656. https://doi.org/10.7554/eLife.31656.001
Yagishita Y, Uruno A, Fukutomi T, Saito R, Saigusa D, Pi J, Fukamizu A, Sugiyama F, Takahashi S, Yamamoto M (2017) Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Rep 18:2030–2044. https://doi.org/10.1016/j.celrep.2017.01.064
Zhang Z, Ma F, Zhou F, Chen Y, Wang X, Zhang H, Zhu Y, Bi J, Zhang Y (2014) Functional polymorphisms of circadian negative feedback regulation genes are associated with clinical outcome in hepatocellular carcinoma patients receiving radical resection. Med Oncol 31:179. https://doi.org/10.1007/s12032-014-0179-1
Zondervan KT, Cardon LR (2004) The complex interplay among factors that influence allelic association. Nat Rev Genet 5:89–100. https://doi.org/10.1038/nrg1270
Acknowledgements
This study was supported by the ‘Development of Korean Medicine Original Technology for Preventive Treatment based on Integrative Big Data’ grant from the Korea Institute of Oriental Medicine (no. KSN2022120).
Author information
Authors and Affiliations
Contributions
Conception or design: M.S.P. Acquisition, analysis, or interpretation of data: M.S.P., S.L., Y.B., J.L., S.S.P., J.H.C., H.J.J., H.R.Y., Drafting the work or revising: M.S.P., J.L., S.S.P., H.J.J., H.R.Y., Final approval of the manuscript: M.S.P., S.L., Y.B., J.L., S.S.P., H.J.C., H.J.J., H.R.Y.
Corresponding author
Ethics declarations
Conflict of interest
Miso S. Park, Siwoo Lee, Younghwa Baek, Juho Lee, Sang-Soo Park, Jung-Hyo Cho, Hee-Jeong Jin, and Ho-Ryong Yoo declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, M.S., Lee, S., Baek, Y. et al. Characteristics of insulin resistance in Korean adults from the perspective of circadian and metabolic sensing genes. Genes Genom 45, 1475–1487 (2023). https://doi.org/10.1007/s13258-023-01443-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-023-01443-0