Abstract
Background
Olive flounder (Paralichthys olivaceus) is one of the major cultured fish species in Asia including Korea. However, the mass mortality of olive flounder caused by various pathogens leads to huge economic loss. The pathogens that lead to fish mortality include parasites, bacteria, and viruses that can cause various kinds of diseases.
Objective
The purpose of this study was to investigate the protein expression patterns in the gills and spleens of olive flounder after artificial infection. We hypothesized that proteomics levels in gills and spleen may be differentially expressed depending on infectious agents.
Methods
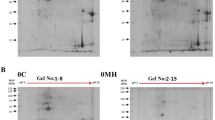
To investigate the expression pattern of proteins in gills and spleens, olive flounders were experimentally infected with VHSV (virus), S. parauberis (bacteria), or M. avidus (pathogenic ciliate). Proteins were extracted from the gills and spleens of infected olive flounder. We used 2-DE analysis with LC–MS/MS to investigate proteome changes in infected olive flounders.
Results
The results of the LC–MS/MS analyses showed different protein expression profiles depending on pathogenic sources and target organs. Proteins related to cytoskeletal structure like keratin, calmodulin and actin were mostly expressed in the infected gills. Proteins involved in the metabolism pathway like glycolysis were expressed mainly in the spleens. The protein profiles of S. parauberis and VHSV infection groups had many similarities, but the profile of the M. avidus infection group was greatly different in the gill and spleen.
Conclusion
Our results indicate that measures according to the characteristics of each pathogen are necessary for disease prevention and treatment of farmed fish.







Similar content being viewed by others
Data availability statement
The raw processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Baeck GW, Kim JH, Gomez DK, Park SC (2006) Isolation and characterization of Streptococcus sp from diseased flounder (Paralichthys olivaceus) in Jeju Island. J Vet Sci 7(1):53–58
Bai SC, Lee SJ (2010) Culture of olive flounder: Korean perspective. In: Daniels H, Watanabe WO (eds) Pratical flatfish culture and stock enhancment. Blackwell Publishing, Hoboken, pp 156–168
Banerjee C, Khatri P, Raman R, Bhatia H, Datta M, Mazumder SJ (2014) Role of calmodulin-calmodulin kinase II, cAMP/protein kinase A and ERK 1/2 on Aeromonas hydrophila-induced apoptosis of head kidney macrophages. PLoS Pathog 10(4):e1004018
Cha I-S, Kwon J, Park S-H, Nho S-W, Jang H-B, Park S-B, Jung T-S (2012) Kidney proteome responses in the teleost fish Paralichthys olivaceus indicate a putative immune response against Streptococcus parauberis. J Proteom 75(17):5166–5175
Cha IS, Kwon J, Mun JY, Park SB, Jang HB, Nho SW, Immunology C (2012) Cathepsins in the kidney of olive flounder, Paralichthys olivaceus, and their responses to bacterial infection. Dev Compar Immunol 38(4):538–544
Cho M-Y, Kim M-S, Choi H-S, Park G-H, Kim J-W, Park M-S, Park MJJ (2008) A statistical study on infectious diseases of cultured olive flounder, Paralichthys olivaceus in Korea. J Fish Pathol 21(3):271–278
Cho MY, Lee UH, Moon CH, Bang JD, Jee BY, Cha SJ, Park JW (2012) Genetically similar VHSV isolates are differentially virulent in olive flounder Paralichthys olivaceus. Dis Aquat Organ 101(2):105–114. https://doi.org/10.3354/dao02503
Food, F. J., & Nations, A. O. o. t. U (2020) The state of world fisheries and aquaculture 2020 Sustainability in action. Aquacult Rep 14:100188
Jacob JT, Coulombe PA, Kwan R, Omary MB (2018) Types I and II keratin intermediate filaments. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a018275
Jung SJ, Kitamura S, Song JY, Oh MJ (2007) Miamiensis avidus (Ciliophora: Scuticociliatida) causes systemic infection of olive flounder Paralichthys olivaceus and is a senior synonym of Philasterides dicentrarchi. Dis Aquat Organ 73(3):227–234. https://doi.org/10.3354/dao073227
Kocmarek AL, Ferguson MM, Danzmann RG (2014) Differential gene expression in small and large rainbow trout derived from two seasonal spawning groups. BMC Genomics 15:57. https://doi.org/10.1186/1471-2164-15-57
Ku NO, Omary MB (2000) Keratins turn over by ubiquitination in a phosphorylation-modulated fashion. J Cell Biol 149(3):547–552
Molle V, Campagna S, Bessin Y, Ebran N, Saint N, Molle G (2008) First evidence of the pore-forming properties of a keratin from skin mucus of rainbow trout (Oncorhynchus mykiss, formerly Salmo gairdneri). Biochem J 411(1):33–40. https://doi.org/10.1042/BJ20070801
Podgorniak T, Milan M, Pujolar JM, Maes GE, Bargelloni L, De Oliveira E, Daverat F (2015) Differences in brain gene transcription profiles advocate for an important role of cognitive function in upstream migration and water obstacles crossing in European eel. BMC Genomics 16:378. https://doi.org/10.1186/s12864-015-1589-y
Retallack H, Okihiro MS, Britton E, Sommeran SV, DeRisi JLJJ (2019) Metagenomic next-generation sequencing reveals Miamiensis avidus (Ciliophora: Scuticociliatida) in the 2017 epizootic of leopard sharks (Triakis semifasciata) in San Francisco Bay. California, USA 55(2):375–386
Rotsch C, Radmacher MJB, j. (2000) Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys J 78(1):520–535
Seo JS, Kim NY, Jeon EJ, Lee NS, Lee EH, Kim MS, Jung SH (2018) Development of a safe antiparasitic against scuticociliates (Miamiensis avidus) in olive flounders: new approach to reduce the toxicity of mebendazole by material remediation technology using full-overlapped gravitational field energy. Parasitol Res. https://doi.org/10.1007/s00436-018-6010-8
Shi X-Z, Zhao X-F, Wang J-X (2008) Molecular cloning and expression analysis of chymotrypsin-like serine protease from the Chinese shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol 25(5):589–597. https://doi.org/10.1016/j.fsi.2008.07.011
Tuli L, Ressom HW (2009) LC-MS based detection of differential protein expression. J Proteomics Bioinform 2:416–438. https://doi.org/10.4172/jpb.1000102
Wang J-J, Sun LJ (2015) Edwardsiella tarda-regulated proteins in Japanese flounder (Paralichthys olivaceus): identification and evaluation of antibacterial potentials. J Proteom 124:1–10
Wang L, Shao C, Xu W, Zhou Q, Wang N, Chen SJ (2017) Proteome profiling reveals immune responses in Japanese flounder (Paralichthys olivaceus) infected with Edwardsiella tarda by iTRAQ analysis. Fish Shelfish Immunol 66:325–333
Woo S-H, Kim J-W, Park S-I, Kim H-J, Lee J-SJJ (2006) Pathogenicity and classification of streptococci isolated from cultured marine fishesd. J Fish Pathol 19(1):17–33
Xing M, Sun X, Zheng F, Qu L, Hong X, Wu S (2011) Proteomic aspects of infection by lymphocystis disease virus in Japanese flounder (Paralichthys olivaceus). Chin J Oceanol Limnol 29(3):603–608
Zhang R, Yang J, Zhu J, Xu X (2009) Depletion of zebrafish Tcap leads to muscular dystrophy via disrupting sarcomere-membrane interaction, not sarcomere assembly. Hum Mol Genet 18(21):4130–4140. https://doi.org/10.1093/hmg/ddp362
Zhou L, Budge SM, Ghaly AE, Brooks MS, Dave DJA (2011) Extraction, purification and characterization of fish chymotrypsin: A review. J Biochem Biotechnol 7(3):104–123
Acknowledgement
This research was a part of a project titled “Omics based on fishery disease control technology development and industrialization (20150242),” funded by the Ministry of Oceans and Fisheries, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, AR., Kim, H., Jeon, KY. et al. Differential proteome profile of gill and spleen in three pathogen-infected Paralichthys olivaceus. Genes Genom 43, 701–712 (2021). https://doi.org/10.1007/s13258-021-01097-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-021-01097-w