Abstract
Pancreatic Cancer is associated with poor treatment outcomes compared to other cancers. High local control rates have been achieved by using hypofractionated stereotactic body radiotherapy (SBRT) to treat pancreatic cancer. Challenges in delivering SBRT include close proximity of several organs at risk (OARs) and target volume inter and intra fraction positional variations. Magnetic resonance image (MRI) guided radiotherapy has shown potential for online adaptive radiotherapy for pancreatic cancer, with superior soft tissue contrast compared to CT. The aim of this study was to investigate the variability of target and OAR volumes for different treatment approaches for pancreatic cancer, and to assess the suitability of utilizing a treatment-day MRI for treatment planning purposes. Ten healthy volunteers were scanned on a Siemens Skyra 3 T MRI scanner over two sessions (approximately 3 h apart), per day over 5 days to simulate an SBRT daily simulation scan for treatment planning. A pretreatment scan was also done to simulate patient setup and treatment. A 4D MRI scan was taken at each session for internal target volume (ITV) generation and assessment. For each volunteer a treatment plan was generated in the Raystation treatment planning system (TPS) following departmental protocols on the day one, first session dataset (D1S1), with bulk density overrides applied to enable dose calculation. This treatment plan was propagated through other imaging sessions, and the dose calculated. An additional treatment plan was generated on each first session of each day (S1) to simulate a daily replan process, with this plan propagated to the second session of the day. These accumulated mock treatment doses were assessed against the original treatment plan through DVH comparison of the PTV and OAR volumes. The generated ITV showed large variations when compared to both the first session ITV and daily ITV, with an average magnitude of 22.44% ± 13.28% and 25.83% ± 37.48% respectively. The PTV D95 was reduced by approximately 23.3% for both plan comparisons considered. Surrounding OARs had large variations in dose, with the small bowel V30 increasing by 128.87% when compared to the D1S1 plan, and 43.11% when compared to each daily S1 plan. Daily online adaptive radiotherapy is required for accurate dose delivery for pancreas cancer in the absence of additional motion management and tumour tracking techniques.
Similar content being viewed by others
Introduction
Pancreatic Cancer is associated with poor treatment outcomes compared to other cancers. Most pancreatic cancer patients present with locally advanced or metastatic disease, with tumours often unresectable due to local invasion of adjacent structures. For patients with resectable disease, the 5 year overall survival is less than 25% [1].
The use of hypofractionated SBRT (1–5 fractions) to treat pancreatic cancer has resulted in high local control rates [2,3,4]. Radiotherapy for pancreatic cancer is challenging due to the close proximity of several organs at risk (OARs) to the pancreas, such as the duodenum, small bowel and stomach. In addition to this, the target volume may have large inter and intra fraction variations due to body contour changes, internal organ motion and changes, and respiratory motion [5]. Respiratory motion alone has the potential to produce intra fraction target motion of the order of 10–20 mm [6]. Day to day interfraction motion can produce variations and displacements of greater than 10 mm for surrounding duodenum, small bowel and stomach in pancreatic cancer radiotherapy [7, 8]. Magnetic resonance guided radiotherapy has also improved the potential of online adaptive radiotherapy for pancreatic cancer [9], with MRI providing high resolution images with superior soft tissue contrast compared to CT [10]. This soft tissue contrast is necessary as there may be considerable interfraction position variation of the target and organs at risk relative to bony anatomy [11, 12], and aids in target delineation, resulting in smaller target volumes and reducing interobserver variation [13].
The advent of adaptive treatment planning has also been required to counter the intra fraction motion within the abdomen which may affect the delivery of radiation to pancreatic cancer. There have been studies attempting to quantify allowable motion in pancreatic cancer radiotherapy treatment, and the clinical effects due to the inter and intra fraction motion [5, 11, 14,15,16].
With the introduction of hybrid MR-guided Linac (MRg-linac) systems, the ability to deliver online MR guided adaptive radiotherapy has become more common [9, 17,18,19,20,21]. These systems facilitate the adaption of treatment plans to accommodate daily position variations, however have strict time constraints as the patient remains on the table in the treatment position whilst plan adaptation occurs. These systems still require treatment planning to occur on a reference CT scan however, with the daily adaptation of treatment plan based off the change in treatment volumes and OAR volumes from the captured MRI guidance scan [22, 23].
The aim of this study was to investigate the variability of target and OAR volumes for different adaptive treatment regimens for pancreatic cancer, and to assess the suitability of utilizing a treatment day MRI for treatment planning purposes, relevant for centres with access to MRI but not an MRg-linac.
Methodology
Ten volunteers were scanned on a Siemens (Erlangen, Germany) Skyra 3 T MRI with a flat radiotherapy couch and coil mounts. Volunteer ages ranged from 25 to 47 with a median age of 35 and body mass index (BMI) ranged from 19.7 to 29.0 with a median BMI of 24.0. Volunteers were scanned in full body vacuum bags for all sessions for setup consistency. Each volunteer was scanned in two sessions per day over 5 days to simulate a potential pancreas SBRT treatment regime, with a daily simulation scan for treatment planning, and a pre treatment scan for treatment. The second imaging session for each day was approximately three hours after the first imaging session. Volunteers were given instructions allowing only one cup of liquid and a small snack between scans, though compliance with these instructions was not strictly enforced being a volunteer study.
Each MRI scanning session consisted of the following scans as presented in Table 1—T1 weighted transverse VIBE with DIXON (16 s exhale breath hold), T2 weighted interleaved TruFISP (3 orthogonal planes) (1 min CINE) and a T1 weighted transverse 4D-MRI (5–6 min). Only the VIBE scan and the 4D MRI scan were used for this study.
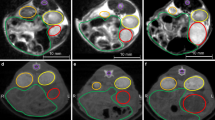
All scans were contoured and planned in the Raystation (RaySearch Laboratories, Stockholm, Sweden) Treatment Planning system (version 10b). The water only images from the T1 weighted VIBE Dixon MRI sequence were used for treatment planning. The first imaging session of the first day (D1S1) was considered the primary planning images for each volunteer. A Radiation Oncologist (RO) contoured a hypothetical tumour volume in the head region of the pancreas, with the last 2 cm of the pancreas used as the gross tumour volume (GTV) volume, as well as the nearby critical organs (ie stomach, duodenum). All other OARs required were contoured by radiation therapists (RTs) and physicists. The 4D MRI was used to generate an internal target volume (ITV) from the GTV, which covered the movement of the GTV over the volunteer breathing cycle. The ITV was transferred to the treatment planning dataset through registration of the exhale phase of the 4D-MRI scan. A 5 mm expansion was applied to the ITV to generate the PTV. The ITV generation using the 4D MRI sequence has been validated against CT previously [24].
Initially, each session on each day was registered to the primary planning images using a rigid registration which focused on the pancreas, vertebral body, and kidney ROIs. The ITV and nearby OARs were assessed for volume change, Dice similarity coefficient (DSC) and Hausdorff distance (HD) for each days mock treatment session compared to the primary planning images.
Three different adaptive treatment methods were considered in this study and are presented in Fig. 1. To enable treatment planning on the MRI images, density overrides were applied to the external contour (1 g/cm3) and vertebral body (1.12 g/cm3) as per the International Commission on Radiation Units and Measurements (ICRU) Report 46 recommendations. The 6MV dual arc VMAT treatment plan was generated following the current departmental protocols on the primary planning images. These clinical goals have been taken from the MASTERPLAN clinical trial (ACTRN12619000409178) and are presented in Tables 2 and 3. This treatment plan was propagated via the rigid registration to all other imaging sessions and recalculated.
The first imaging session of each day (S1) was also replanned as would occur with a daily adaptive treatment plan, and corresponding treatment (S2) later that day. Treatment plan quality consistency was maintained through the use of templates for VMAT optimisation objectives and comparison of clinical goals in the initial D1S1 treatment plan. This method was utilised as it was the potential clinical translation of this work, with patients undergoing a daily MRI simulation session followed by radiotherapy on a conventional linear accelerator. The treatment plan generated on the first session of each day was propagated to the second session and recalculated, with this accumulated to indicate daily replanning and treatment. In addition, a treatment plan was generated on the second session of each day, with these accumulated to indicate online treatment adaptation. This was completed to ensure that the clinical goals were able to be met on that dataset. Both instances were compared with the treatment plan generated on the primary planning images through DVH comparison of the clinical goals presented in Tables 2 and 3.
Results
The ITV varied across all volunteers and for both sets of comparisons, with the ITV variations for each volunteer presented in Fig. 2. On average, the ITV variation was − 0.85% ± 23.68% across all volunteers. ITV variations over the course of the mock treatment were quite high for both comparison with the baseline scan and the daily scan for each volunteer.
Contour assessment was also undertaken considering the GTV, ITV and the surrounding organs at risk for the second imaging session of each day for both when a single reference scan is considered or a daily reference scan. The results for the average across the 10 volunteer dataset, considering the DSC, mean HD agreement and the volume change for both comparisons is shown in Table 4. The DSC results were improved when considering a daily reference as opposed to a single reference for all volumes considered. When the volume change is considered, the absolute volume changes are also smaller when a daily reference is considered. The external volume DSC is similar for both when compared to a D1S1 scan and to a daily scan, with a result of 0.959 ± 0.021 and 0.968 ± 0.009 respectively. All OARs had poor DSC, as well as large amounts of volume change for all comparisons.
Table 5 displays the mock treatment plan comparison as a whole, with each dose accumulation methodology averaged. Figure 3 displays the average results for the rigid dose accumulation for both comparison to the D1S1 plan and the daily plan. Online adaptive generally allowed clinical goals to be met, though large variations are seen for the small bowel and stomach V30 for the online adaptive comparison due to the small absolute volumes receiving 30 Gy for these OAR in the treatment plans.
The target volume coverage on average was reduced when considering the accumulated dose for both mock treatment with either a D1S1 reference plan or a daily reference plan as can be seen in Fig. 3. The PTV40 D95 was on average reduced by − 23.3% when both sets of reference plans are considered, with small bowel and duodenum V30 increasing for both sets of plan comparison. The duodenum V30 increased by 1.48% compared to the D1S1 reference plan, and 53.91% compared to the daily reference plan, while the small bowel V30 increased by 128.87% and 43.11% respectively.
Figures 4 and 5 display the DVH differences for the PTV40 D95 and the small bowel D0.5 cc for both sets of plan comparisons for all volunteers. On a per volunteer basis, these differences varied between plan comparisons, with some volunteers benefitting from the daily plan adaption. Though on average, as seen in Fig. 3, these parameters may show similar variations, the differences are much more varied on a per volunteer basis.
Discussion
This study considered the variation in internal anatomy between daily imaging sessions and over different days, and the variation in dose to target volume and nearby organs at risk for a simulated pancreas SBRT radiotherapy treatment. MR imaging was used for this comparison, with volunteers undertaking twice daily MRI simulation scans over 5 days within a 2 week period on a radiotherapy MRI simulator to simulate a 5 fraction treatment regime, with the first imaging session of each day a simulation session, and the second imaging session a mock treatment session, ie pre treatment imaging. A limitation of this study is that it is a volunteer study considering only a simulated tumour in the head of the pancreas and does not contain any patient data where the tumour location may vary.
The variation in internal volume anatomy has been studied previously. Heerkens et al. [25] studied MRI based tumor motion characterization, using sagittal and coronal cine MRIs of 60 s in 15 pancreatic cancer patients to quantify tumor motion. This study found tumor motion was largest in the craniocaudal direction, with an average amplitude of 15 mm and with a range of 6–34 mm. The average in the anterior–posterior direction was 5 mm, with range 1–13 mm, and in the lateral direction an average of 3 mm, range 2–5 mm. Alam et al. [26] looked at a pre treatment, verification and post treatment MRI for each abdominally compressed pancreas cancer patient treatment fraction, finding median (max) interfraction deformation for the stomach/duodenum and small bowel of 6.1 (25.8) mm and 7.9 (40.5) mm respectively and median intrafraction deformation was 5.5 (22.6) mm and 8.2 (37.8) mm respectively. These large variations are similar for both inter and intra fraction motion, indicating large displacements for both even with abdominal compression. This study also reported median DSC similarity scores for the duodenum-stomach and small bowel of 0.7, which is similar to that reported in this study.
It had been considered that a daily replanning exercise may be sufficient for this cohort of patient, especially in the absence of an MR guided linac. From Table 2, the average DSC, HD and volume change had improved results for all volumes compared in the daily scans when compared to the results which compared the volumes back to the D1S1 reference scan. However the dosimetric results from Table 5 for the daily plans recalculated on the treatment scan of the day showed large variations in dose coverage and OAR dose. Poor compliance with instructions for food and drink between imaging sessions may have contributed to the large variations in organ location and volume within this study for the daily replan. Additionally, this cohort of patients would potentially be treated with an empty stomach each day for consistency, which did not occur in this volunteer study. This may have contributed to the day to day variations seen between scans. Some volunteers would have benefited from the daily scans though, as can be seen from the comparisons in Figs. 4 and 5 regarding the PTV D95 and the OAR DVH differences, with approximately 40% of volunteers showing better agreement in regards to DVH analysis with the appropriate reference plan.
Large variations in the ITV were seen in Fig. 2 and Table 4. Additionally in Table 4, variations in volume were seen for the GTV. As the GTV was re-contoured for each imaging session, intra-observer contour variation may have contributed to the ITV variation as well, with this being a potential source of error [27, 28]. Additionally, a lack of motion management may also contribute to this ITV variation. This shows that a 4D ITV method may not be sufficient for these patients, with other motion management techniques [29, 30] such as gated techniques, implanted markers/tumour tracking or compression techniques able to reduce the variation in ITV on a daily basis, and potentially enable more accurate delivery of dose to the tumour whilst maintaining OAR doses [31, 32]. However this would not necessarily reduce any interfraction motion of the OAR, which occurred throughout this study for all volunteers. Fiducial markers implanted in the target volume would be useful for determining the interfraction and intrafraction motion of the target. Though this study was able to visualize the target volume, fiducial markers would aid the accuracy of the registration back to reference images and plan.
When comparing the dose delivery considering only a single baseline plan, as well as comparing to a daily plan, the target dose was deficient in most days considered due to anatomical changes. These variations included both nearby OAR variations, as well as changes in ITV generation due to the variability in volunteer breathing, which generated a different daily PTV volume for the day. It should be considered that if the ITV was smaller, that the previous dose coverage should still be sufficient for target coverage, however the average DSC of 0.594 ± 0.158, average HD of 3.66 mm ± 1.69 mm and average volume variation of − 0.25% ± 19.70% would indicate that the ITV volume and position varied when compared to the D1S1 ITV. For the daily plan, these results were only slightly improved, with an average DSC of 0.615 ± 0.141, average HD of 3.41 mm ± 1.67 mm and average volume variation of 8% ± 18.95%.
The conventional method of simulation and planning based off a single reference scan is not appropriate for this treatment site for a hypofractionated SBRT treatment as per the variations seen in this study. Scanning on the day and completing treatment planning with the scan on the day may be appropriate for some patients with appropriate motion management but still may have large variations in internal organ anatomy between the scan on the day and the treatment scan, particularly if strict protocols are not followed regarding intake. Online adaptive treatment is the best option for ensuring target dose coverage whilst minimizing dose to nearby OARs, particularly the duodenum, small bowel and stomach [33]. This was seen from the results presented in Table 5, though some large variations in OAR dose are presented due to the low absolute dose for these DVH parameters. This methodology is current best practice and has been utilized with hybrid MR-guided linacs [8, 19,20,21, 34], allowing delivery of hypofractionated SBRT treatment for pancreatic cancer using real time adaptation of the dose distribution to account for day to day variations in organ shapes and position. This is currently not possible with conventional linear accelerators however, and a daily MRI scan in conjunction with daily plan adaptation and additional motion management techniques and tracking may allow more accurate treatment of pancreas SBRT whilst reducing dose to nearby OARs.
Conclusion
Daily online adaptive radiotherapy is required for accurate dose delivery for pancreas cancer in the absence of additional motion management and tumour tracking techniques.
Data availability
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
References
Mahadevan A, Moningi S, Grimm J, Li XA, Forster KM, Palta M et al (2021) Maximizing tumor control and limiting complications with stereotactic body radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys 110:206–216
Courtney PT, Paravati AJ, Atwood TF, Raja N, Zimmerman CT, Fanta PT et al (2021) Phase I trial of stereotactic body radiation therapy dose escalation in pancreatic cancer. Int J Radiat Oncol Biol Phys 110:1003–1112
de Geus SW, Eskander MF, Kasumova GG, Ng SC, Kent TS, Mancias JD et al (2017) Stereotactic body radiotherapy for unresected pancreatic cancer: a nationwide review. Cancer 123:4158–4167
Reyngold M, Parikh P, Crane CH (2019) Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol 14:95
van der Horst A, Houweling AC, van Tienhoven G, Visser J, Bel A (2017) Dosimetric effects of anatomical changes during fractionated photon radiation therapy in pancreatic cancer patients. J Appl Clin Med Phys 18:142–151
Akimoto M, Nakamura M, Nakamura A, Mukumoto N, Kishi T, Goto Y et al (2017) Inter-and intrafractional variation in the 3-dimensional positions of pancreatic tumors due to respiration under real-time monitoring. Int J Radiat Oncol Biol Phys 98:1204–1211
Liu F, Erickson B, Peng C, Li XA (2012) Characterization and management of interfractional anatomic changes for pancreatic cancer radiotherapy. Int J Radiat Oncol Biol Phys 83:e423–e429
Magallon-Baro A, Milder MT, Granton PV, Nuyttens JJ, Hoogeman MS (2021) Comparison of daily online plan adaptation strategies for a cohort of pancreatic cancer patients treated with SBRT. Int J Radiat Oncol Biol Phys 111:208–219
Boldrini L, Cusumano D, Cellini F, Azario L, Mattiucci GC, Valentini V (2019) Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: state of the art, pearls and pitfalls. Radiat Oncol 14:1–6
Paganelli C, Whelan B, Peroni M, Summers P, Fast M, Van de Lindt T et al (2018) MRI-guidance for motion management in external beam radiotherapy: current status and future challenges. Phys Med Biol 63:22TR03
Jayachandran P, Minn AY, Van Dam J, Norton JA, Koong AC, Chang DT (2010) Interfractional uncertainty in the treatment of pancreatic cancer with radiation. Int J Radiat Oncol Biol Phys 76:603–607
Nakamura M, Akimoto M, Ono T, Nakamura A, Kishi T, Yano S et al (2015) Interfraction positional variation in pancreatic tumors using daily breath-hold cone-beam computed tomography with visual feedback. J Appl Clin Med Phys 16:108–116
Gurney-Champion OJ, Versteijne E, van der Horst A, Lens E, Rütten H, Heerkens HD et al (2017) Addition of MRI for CT-based pancreatic tumor delineation: a feasibility study. Acta Oncol 56:923–930
Ding Y, Campbell WG, Miften M, Vinogradskiy Y, Goodman KA, Schefter T et al (2019) Quantifying allowable motion to achieve safe dose escalation in pancreatic SBRT. Pract Radiat Oncol 9:e432–e442
Niedzielski JS, Liu Y, Ng SS, Martin RM, Perles LA, Beddar S et al (2021) Dosimetric uncertainties resulting from interfractional anatomic variations for patients receiving pancreas stereotactic body radiation therapy and cone beam computed tomography image guidance. Int J Radiat Oncol Biol Phys 111:1298–1309
Feng M, Balter JM, Normolle D, Adusumilli S, Cao Y, Chenevert TL et al (2009) Characterization of pancreatic tumor motion using cine MRI: surrogates for tumor position should be used with caution. Int J Radiat Oncol Biol Phys 74:884–891
Chow PE, Chu FI, Agazaryan N, Cao M, Tyran M, Yang Y et al (2021) Dosimetric quality of online adapted pancreatic cancer treatment plans on an MRI-guided radiation therapy system. Adv Radiat Oncol 6:100682
Ermongkonchai T, Khor R, Muralidharan V, Tebbutt N, Lim K, Kutaiba N et al (2022) Stereotactic radiotherapy and the potential role of magnetic resonance-guided adaptive techniques for pancreatic cancer. World J Gastroenterol 28:745
Hassanzadeh C, Rudra S, Bommireddy A, Hawkins WG, Wang-Gillam A, Fields RC et al (2021) Ablative five-fraction stereotactic body radiation therapy for inoperable pancreatic cancer using online MR-guided adaptation. Adv Radiat Oncol 6:100506
Placidi L, Romano A, Chiloiro G, Cusumano D, Boldrini L, Cellini F et al (2020) On-line adaptive MR guided radiotherapy for locally advanced pancreatic cancer: clinical and dosimetric considerations. Tech Innov Patient Support Radiat Oncol 15:15–21
Nierer L, Eze C, da Silva MV, Braun J, Thum P, von Bestenbostel R et al (2022) Dosimetric benefit of MR-guided online adaptive radiotherapy in different tumor entities: liver, lung, abdominal lymph nodes, pancreas and prostate. Radiat Oncol 17:1–14
Paulson ES, Ahunbay E, Chen X, Mickevicius NJ, Chen G-P, Schultz C et al (2020) 4D-MRI driven MR-guided online adaptive radiotherapy for abdominal stereotactic body radiation therapy on a high field MR-Linac: implementation and initial clinical experience. Clin Transl Radiat Oncol 23:72–79
Winkel D, Bol GH, Kroon PS, van Asselen B, Hackett SS, Werensteijn-Honingh AM et al (2019) Adaptive radiotherapy: the Elekta Unity MR-linac concept. Clin Transl Radiat Oncol 18:54–59
Oar A, Liney G, Rai R, Deshpande S, Pan L, Johnston M et al (2018) Comparison of four dimensional computed tomography and magnetic resonance imaging in abdominal radiotherapy planning. Phys Imaging Radiat Oncol 7:70–75
Heerkens HD, van Vulpen M, van den Berg CA, Tijssen RH, Crijns SP, Molenaar IQ et al (2014) MRI-based tumor motion characterization and gating schemes for radiation therapy of pancreatic cancer. Radiother Oncol 111:252–257
Alam S, Veeraraghavan H, Tringale K, Amoateng E, Subashi E, Wu AJ et al (2022) Inter-and intrafraction motion assessment and accumulated dose quantification of upper gastrointestinal organs during magnetic resonance-guided ablative radiation therapy of pancreas patients. Phys Imaging Radiat Oncol 21:54–61
Cattaneo GM, Passoni P, Sangalli G, Slim N, Longobardi B, Mancosu P et al (2010) Internal target volume defined by contrast-enhanced 4D-CT scan in unresectable pancreatic tumour: evaluation and reproducibility. Radiother Oncol 97:525–529
Park S, Chu L, Fishman E, Yuille A, Vogelstein B, Kinzler K et al (2020) Annotated normal CT data of the abdomen for deep learning: challenges and strategies for implementation. Diagn Interv Imaging 101:35–44
Brandner ED, Chetty IJ, Giaddui TG, Xiao Y, Huq MS (2017) Motion management strategies and technical issues associated with stereotactic body radiotherapy of thoracic and upper abdominal tumors: a review from NRG oncology. Med Phys 44:2595–2612
Campbell WG, Jones BL, Schefter T, Goodman KA, Miften M (2017) An evaluation of motion mitigation techniques for pancreatic SBRT. Radiother Oncol 124:168–173
Arumugam S, Young T, Johnston M, Pavey D, Lee M (2022) The delivered dose assessment in pancreas SBRT with the target position determined using an in-house position monitoring system. Front Oncol. https://doi.org/10.3389/fonc.2022.1009916
Vinogradskiy Y, Goodman KA, Schefter T, Miften M, Jones BL (2019) The clinical and dosimetric impact of real-time target tracking in pancreatic SBRT. Int J Radiat Oncol Biol Phys 103:268–275
El-Bared N, Portelance L, Spieler BO, Kwon D, Padgett KR, Brown KM et al (2019) Dosimetric benefits and practical pitfalls of daily online adaptive MRI-guided stereotactic radiation therapy for pancreatic cancer. Pract Radiat Oncol 9:e46–e54
Tyagi N, Liang J, Burleson S, Subashi E, Scripes PG, Tringale KR et al (2021) Feasibility of ablative stereotactic body radiation therapy of pancreas cancer patients on a 1.5 Tesla magnetic resonance-linac system using abdominal compression. Phys Imaging Radiat Oncol 19:53–59
Acknowledgements
The authors would like to thank Jason Dowling, Lois Holloway and David Thwaites for advice and review of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors received no funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest. Liverpool Cancer Therapy Centre has a master research agreement with Siemens, unrelated to this study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of data.
Research involving humans and animal participants
Volunteer data was acquired with local ethics committee approval, HREC/15/LPOOL/506.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Young, T., Lee, M., Johnston, M. et al. Assessment of interfraction dose variation in pancreas SBRT using daily simulation MR images. Phys Eng Sci Med 46, 1619–1627 (2023). https://doi.org/10.1007/s13246-023-01324-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-023-01324-6