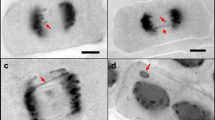
Abstract
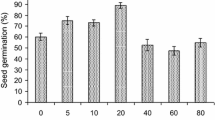
Dose-responses of germination and seedling growth of plants namely Amaranthus blitum, Cajanus cajan, Eleusine coracana, Guizotia abyssinica, Lathyrus sativus and Lycopersicon esculentum obtained following γ-irradiation of seeds at a wide range of doses established G. abyssinica (LD50 = 0.32; D50 = 0.17 kGy) as highly radio-sensitive species. Seed-irradiation caused oxidative stress to the emerging seedlings that was evident by the dose-dependent increase in the generation of reactive oxygen species (ROS: H2O2, O2 •−, •OH), which in turn also showed good correlation with the dose-dependent induction of cell death, lipid peroxidation, protein oxidation, protein and non-protein thiols, and antioxidant enzymes activities, in the leaf tissue of G. abyssinica seedlings. The above biochemical endpoints could, therefore, serve as biomarkers of the previous exposure of seeds to ionizing radiation. Furthermore, the highest ineffective threshold (HITD = 0.019 kGy) and the lowest effective threshold (LETD = 0. 021 kGy) doses of ionizing radiation were determined on the basis of induction of cells with chromosome aberration (CA) or micronucleus (MN) in root meristems of the germinating irradiated seeds. The present G. abyssinica genotoxicity assay thus validated the non-linear threshold (NLT) dose-response to ionizing radiation as opposed to the prevailing linear no-threshold (LNT) dose-response concept.



Similar content being viewed by others
References
Achary VMM, Parinandi NL, Panda BB. Aluminium induces oxidative burst, cell wall NADH peroxidase activity and DNA damage in root cells of Allium cepa L. Environ Mol Mutagen. 2012;53:550–60.
Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–6.
Al-Rumaih MM, Al-Rumaih MM. Influence of ionizing radiation on antioxidant enzymes in three species of Trigonella. Am J Environ Sci. 2008;4:151–6.
Asada K. Ascorbate peroxidase - a hydrogen peroxide-scavenging enzymes in plants. Physiol Plant. 1992;85:235–41.
Ballarini F, Ottolenghi A. Chromosome aberrations as biomarkers of radiation exposure: modelling basic mechanisms. Adv Space Res. 2003;31:1557–68.
Bauchinger M. Quantification of low-level radiation exposure by conventional chromosome aberration analysis. Mutat Res. 1995;339:77–89.
Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87.
Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–54.
Bressani R, Sánchez-Maroquín A, Morales E. Chemical composition of grain Amaranth cultivars and effects of processing on their nutritional quality. Food Rev Int. 1992;8:23–49.
Brechignac F. Protecting the environment against ionizing radiation: the path proposed by “ICRP”, its origin and analysis. In: Arapis G, Goncharova N, Baveye P, editors. Ecotoxicology, ecological risk assessment and multiple stressor. Dordrecht: Springer; 2006. p. 41–5.
Calabrese EJ. Origin of the linearity no-threshold (LNT) dose-response concept. Arch Toxicol. 2013;87:1621–33.
Calabrese EJ, Bachmann KA, Bailer AJ, et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–8.
Chance B, Maehly AC. Assay of catalase and peroxidases. Method Enzymol. 1995;2:764–77.
Cheeseman JM. Hydrogen peroxide and plant stress: a challenging relationship. Plant Stress. 2007;1:4–15.
Chen GX, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–98.
Conger AD, Stevenson HQ. A correlation of seedling height and chromosomal damage in irradiated barley seeds. Radiat Bot. 1969;9:1–14.
Dagne K, Heneen WK. The karyotype and nucleoli of Guizotia abyssinica (Compositae). Hereditus. 1992;117:73–83.
Dimova EG, Bryant PE, Chankova SG. “Adaptive response”—some underlying mechanisms and open questions. Genet Mol Biol. 2008;31:396–408.
Esnault MA, Legue F, Chenal C. Ionizing radiation: advances in plant response. Environ Exp Bot. 2010;68:231–7.
Foyer CH, Halliwell B. The presence of glutathione and glultathione reducase in chloroplast: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–5.
Geras’kin SA, Oudalova AA, Kim JK, Dikarev VG, Dikareva NS. Cytogenetic effect of low dose gamma-radiation in Hordeum vulgare seedlings: non-linear dose-effect relationship. Radiat Environ Biophys. 2007;46:31–41.
Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30.
Gomez KA, Gomez AA. Statistical procedures for agricultural research. 2nd ed. New York: Wiley; 1984.
Goud JV, Nayar KM, Rao MG. Radio-sensitivity in ragi (Eleusine coracana). Can J Genet Cytol. 1969;11:254–65.
Grant WF, Owens ET. Lycopersicon assays of chemical/radiation genotoxicity for the study of environmental mutagens. Mutat Res. 2002;511:207–37.
Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc Royal Soc A. 1934;147:332–51.
Hiremath SC, Murthy HN, Salimath SS. Quantitative nuclear DNA differences associated with genome evolution in Guizotia (Compositae). Genetica. 1992;85:241–7.
Iglesias-Andreu LG, Octavio-Aguilar P, Bello-Bello J. Current importance and potential use of low doses of gamma radiation in forest species. In: Adrovic F, editor. Gamma radiation. 2012. p. 263–80.
Ishikawa T, Yoshimura K, Sakai K, Tamoi M, Takeda T, Shigeoka S. Molecular characterization and physiological role of a glyoxisome-bound ascorbate peroxidase from spinach. Plant Cell Physiol. 1998;39:23–34.
Jan S, Parween T, Siddiqi TO, Mahmooduzzafar X. Anti-oxidant modulation in response to gamma radiation induced oxidative stress in developing seedlings of Psoralea corylifolia L. J Environ Radioact. 2012;113:142–9.
Jimenez A, Hernandez JA, del Rio LA, Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997;114:275–84.
Juchimiuk-Kwasniewska J, Brodziak L, Maluszynska J. FISH in analysis of gamma ray-induced micronuclei formation in barley. J Appl Genet. 2011;52:23–9.
Kumar A, Haidar ZA. Mutagenic sensitivity of Brassica juncea L. to gamma-rays, EMS alone and in combination. J Nucl Agric Biol. 1998;27:275–9.
Larsson CM. Biological basis for protection of the environment. Ann ICRP. 2012;41:208–17.
Lee MH, Moon YR, Chung BY, Kim JS, Lee KS, Cho JY, et al. Practical use of chemical probes for reactive oxygen species produced in biological systems by γ-irradiation. Radiat Phys Chem. 2009;78:323–7.
Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–22.
Mello Filho AC, Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984;781:56–63.
Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett. 2009;12:75–92.
NAS/NRC. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2, Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, Board on Radiation Effects Research, Division on Earth and Life Studies, National Research Council. Washington DC: National Academies Press; 2006.
Natarajan AT, Palitti F. DNA repair and chromosomal alterations. Mutat Res. 2008;657:3–7.
Nayyar H, Gupta D. Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environ Exp Bot. 2006;58:106–13.
Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–79.
Panda BB, Achary VMM, Mahanty S, Panda KK. Plant adaptation to abiotic and genotoxic stress: relevance to climate change and evolution. In: Tuteja N, Gill SS, editors. Climate change and abiotic stress tolerance. Weinheim: Wiley-VCH; 2013. p. 251–94.
Patra J, Panda BB. A comparisons of biochemical responses to oxidative and metal stress in seedlings of barley Hordeum vulgare L. Environ Pollut. 1998;101:99–105.
Panwar P, Saini RK, Sharma N, Yadav D, Kumar A. Efficiency of RAPD, SSR and cytochrome P450 gene based markers in accessing genetic variability amongst finger millet (Eleusine coracana) accessions. Mol Biol Rep. 2010;37:4075–82.
Plant list. The plant list - a working list of all plant species, Version 1.1, 2014. http://www.theplantlist.org/. Accessed 15 Jul 2014.
Rajpurohit TS. Diseases of niger and their management. Plant Sci Feed. 2011;1:19–22.
Rathnam CKM, Das VSR. Nitrate metabolism in relation to the aspartate-type C-4 pathway of photosynthesis in Eleusine coracana. Can J Bot. 1974;52:2599–605.
Sah NK, Pramanik S, Raychaudhuri S. Peroxidase changes in barley induced by ionizing and thermal radiation. Int J Radiat Biol. 1996;69:107–11.
Saha P, Das D, Roy S, Chakrabarti A, Raychaudhur SS. Effect of gamma irradiation on metallothionein protein expression in Plantago ovata Forsk. Int J Radiat Biol. 2013;89:88–96.
Saxena KB. Genetic improvement of pigeon pea - a review. Trop Plant Biol. 2008;1:159–78.
Sedlak J, Linsay RH. Estimation of total, protein bound, and non protein sulfhydril groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205.
Sharma AK, Sharma A. Chromosome techniques - theory and practice. 3rd ed. London: Butterworth-Heinemann; 1980.
Tripathi R, Kumar G. Comparative effect of ageing and gamma irradiation on the somatic cells of Lathyrus sativus L. J Cent Eur Agric. 2010;11:437–42.
Truta LA, Hofmann W, Cosma C, Radulescu D. Health effects attributed to radon from the perspective of the linear no-threshold hypothesis. Rom J Phys. 2013;58:S280–90.
Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13–22.
Ulsh B, Hinton TG, Congdon JD, Dugan LC, Whicker FW, Bedford JS. Environmental biodosimetry: a biologically relevant tool for ecological risk assessment and biomonitoring. J Environ Radioact. 2003;66:121–39.
UNSCEAR. Sources and effects of ionizing radiation, Repot of United Nations Scientific Committee on Effect of Atomic Radiation to General Assembly, Vol. II, NewYork: United Nations, 2011.
USGAO. Radiation Standards: scientific basis inconclusive, and EPA and NRC disagreement continues. Report to the Honourable Pete Domenici, U. S. Senate., United States General Accounting Office Washington, DC.GAO/RCED-00-152. 2000.
Uzilday B, Turkan I, Sekmen AH, Ozgur R, Karakaya HC. Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci. 2012;182:59–70.
Velentin J. A framework for assessing the impact of ionising radiation on non-human species. Publication 91. Ann ICRP. 2003;33:207–66.
Vignard J, Mirey G, Salles B. Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiother Oncol. 2013;108:362–9.
Willekens H, Chamnongpol S, Davey M, Langebartels MC, Van Montagu M, Inze D, et al. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997;16:4806–16.
Zaichkina SI, Rozanova OM, Aptikaeva GF, Achmadieva AC, Klokov DY. Low doses of gamma-radiation induce nonlinear dose responses in mammalian and plant cells. Nonlinear Biol Toxicol Med. 2004;2:213–21.
Zaka R, Chenal C, Misset MT. Study of external low irradiation dose effects on induction of chromosome aberrations in Pisum sativum root tip meristem. Mutat Res. 2002;517:87–99.
Zaka R, Vandecasteele CM, Misset MT. Effects of low chronic doses of ionizing radiation on antioxidant enzymes and G6PDH activities in Stipa capillata (Poaceae). J Exp Bot. 2002;53:1979–87.
Acknowledgments
This research was sponsored by a grant received from Bhabha Atomic Research Centre (BARC), Department of Atomic Energy, Government of India, Mumbai through a XIth Plan grant under the MoU between the authorities of BARC and Berhampur University (BU). The authors thank the BU authorities for providing infrastructure and administrative support to carry out this research project. Financial support in form of Rajiv Gandhi National Fellowship to DG and AV, and Emeritus Fellowship to BBP awarded by the University Grant Commission, New Delhi is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golari, D., Venugopal, A., Venu-Babu, P. et al. Oxidative stress and non-linear threshold (NLT) genotoxic dose responses to ionizing radiation in niger, Guizotia abyssinica (L.f.) Cass. Nucleus 57, 175–184 (2014). https://doi.org/10.1007/s13237-014-0126-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-014-0126-8