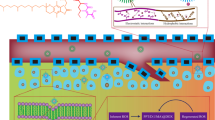
Abstract
Chemotherapy is the main strategy for inhibiting the tumor growth. However, during the therapy, the side effect of anti-cancer drug will injure normal cells. For reducing the toxic of the free drug, stimuli responsive nanoparticles (NPs) have been prepared base on the pathological acidic environment around tumor for safe and efficient anti-tumor applications. In this study, we fabricated the phenylboronic acid (PBA) modified monomethoxy polyethylene glycol (MEPG) for encapsulating doxorubicin (DOX NPs). In the drug delivery system (DDS), the pH-responsive boric ester could be stable during drug delivery, and enhance DOX rapidly release in tumor lesion under the acidic stimuli. In vitro studies further demonstrated that DOX NPs could efficiently endocytose by tumor cells, and exhibit the similar anti-tumor activity as the free DOX resulting from the pH-responsive drug release profiles under tumor acidic stimuli. In summary, DOX NPs could be a feasible candidate to improve the safe and efficient DOX delivery for improving the potential anti-tumor applications.
Graphical abstract








Similar content being viewed by others
References
L. Zhu, Y. Zhong, S. Wu, M. Yan, Y. Cao, N. Mou, G. Wang, D. Sun, W. Wu, Cell membrane camouflaged biomimetic nanoparticles: Focusing on tumor theranostics. Mater. Today. Bio. 14, 100228 (2022)
L. Liu, R. Wang, C. Wang, J. Wang, L. Chen, J. Cheng, Light-triggered release of drug conjugates for an efficient combination of chemotherapy and photodynamic therapy. Biomater. Sci. 6, 5 (2018)
C. Friesen, I. Herr, P.H. Krammer, K.M. Debatin, Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat. Med. 2, 5 (1996)
H.M. Kuerer, L.A. Newman, T.L. Smith, F.C. Ames, K.K. Hunt, K. Dhingra, R.L. Theriault, G. Singh, S.M. Binkley, N. Sneige, T.A. Buchholz, M.I. Ross, M.D. McNeese, A.U. Buzdar, G.N. Hortobagyi, S.E. Singletary, Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J. Clin. Oncol. 17, 2 (1999)
F.M. Muggia, J.D. Hainsworth, S. Jeffers, P. Miller, S. Groshen, M. Tan, L. Roman, B. Uziely, L. Muderspach, A. Garcia, A. Burnett, F.A. Greco, C.P. Morrow, L.J. Paradiso, L.J. Liang, Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J. Clin. Oncol. 15, 3 (1997)
O. Tacar, P. Sriamornsak, C.R. Dass, Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 65, 2 (2013)
X. Shuai, H. Ai, N. Nasongkla, S. Kim, J. Gao, Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J. Control. Release. Off: J. Control. Release Soc. 98, 3 (2004)
D. Kim, E.S. Lee, K. Park, I.C. Kwon, Y.H. Bae, Doxorubicin loaded pH-sensitive micelle: antitumoral efficacy against ovarian A2780/DOXR tumor. Pharm. Res. 25, 9 (2008)
J. Liu, D. Tu, J. Dancey, L. Reyno, K.I. Pritchard, J. Pater, L.K. Seymour, Quality of life analyses in a clinical trial of DPPE (tesmilifene) plus doxorubicin versus doxorubicin in patients with advanced or metastatic breast cancer: NCIC CTG Trial MA.19. Breast. Cancer. Res. Treat. 100, 3 (2006)
R. Langer, D.A. Tirrell, Designing materials for biology and medicine. Nature 428, 6982 (2004)
Y. Minamitake, R. Gref, M.T. Peracchia, V. Trubetskoy, V. Torchilin, R. Langer, Biodegradable long-circulating polymeric nanospheres. Science 263, 1600–1603 (1994)
B-BC Youan, Impact of nanoscience and nanotechnology on controlled drug. Nanomedicine, 3 (2008)
J. Chan, L. Zhang, F.X. Gu, J.-W. Rhee, A.Z. Wang, A.F. Radovic-Moreno et al., Self assembled lipid-polymer hybrid nanoparticles a robust drug delivery. ACS Nano 2, 1696–1702 (2008)
F. Alexis, E.M. Pridgen, R. Langer, O.C. Farokhzad, Nanoparticle technologies for cancer therapy. Handbook. Exp. Pharmacol. 197 (2010)
O.C. Farokhzad, R. Langer, Impact of nanotechnology on drug delivery. ACS Nano 3, 1 (2009)
C. Sun, L. Zhou, M. Gou, S. Shi, T. Li, J. Lang, Improved antitumor activity and reduced myocardial toxicity of doxorubicin encapsulated in MPEG-PCL nanoparticles. Oncol. Rep. 35, 6 (2016)
J.Z. Du, X.J. Du, C.Q. Mao, J. Wang, Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 133, 44 (2011)
X. Li, B.Y. Zheng, M.R. Ke, Y. Zhang, J.-D. Huang, J. Yoon, A tumor-pH-responsive supramolecular photosensitizer for activatable photodynamic therapy with minimal in vivo skin phototoxicity. Theranostics. 7, 10 (2017)
J. Liao, H. Zheng, Z. Fei, B. Lu, H. Zheng, D. Li, X. Xiong, Y. Yi, Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int. J. Biological. Macromol. 113, 737–747 (2018)
H. Ma, Y. Liu, M. Shi, X. Shao, W. Zhong, W. Liao, M.M.Q. Xing, Theranostic, pH-responsive, doxorubicin-loaded nanoparticles inducing active targeting and apoptosis for advanced gastric cancer. Biomacromol 16, 12 (2015)
X. Zhang, M. Zhao, N. Cao, W. Qin, M. Zhao, J. Wu, D. Lin, Construction of a tumor microenvironment pH-responsive cleavable PEGylated hyaluronic acid nano-drug delivery system for colorectal cancer treatment. Biomater, Science. 8, 7 (2020)
W. Wu, L. Luo, Y. Wang, Q. Wu, H.B. Dai, J.S. Li, C. Durkan, N. Wang, G.-X. Wang, Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics 8, 11 (2018)
T. Chen, W. Wu, H. Xiao, Y. Chen, M. Chen, J. Li, Intelligent drug delivery system based on mesoporous silica nanoparticles coated with an ultra-pH-sensitive gatekeeper and poly(ethylene glycol). ACS Macro. Lett. 5, 1 (2016)
S. Li, W. Wu, K. Xiu, F. Xu, Z. Li, J. Li, Doxorubicin loaded pH-responsive micelles capable of rapid intracellular drug release for potential tumor therapy. J. Biomed. Nanotechnol. 10(8), 1480–1489 (2014)
S. Li, Z. Zhao, W. Wu, C. Ding, J. Li, Dual pH-responsive micelles with both charge-conversional property and hydrophobic–hydrophilic transition for effective cellular uptake and intracellular drug release. Polym. Chem. 7, 12 (2016)
L. Luo, W. Wu, D. Sun, H.B. Dai, Y. Wang, Y. Zhong, J.X. Wang, A. Maruf, D. Nurhidayah, X.J. Zhang, Y. Wang, G.-X. Wang, Acid-activated melittin for targeted and safe antitumor therapy. Bioconjug. Chem. 29, 9 (2018)
W. Wu, M. Chen, J. Wang, Q. Zhang, S. Li, Z. Lin, J. Li, Nanocarriers with dual pH-sensitivity for enhanced tumor cell uptake and rapid intracellular drug release. RSC Adv. 4, 58 (2014)
W. Wu, J. Wang, Z. Lin, X. Li, J. Li, Tumor-acidity activated surface charge-conversion of polymeric nanocarriers for enhanced cell adhesion and targeted drug release. Macromol. Rapid Commun. 35, 19 (2014)
W. Wu, Q. Zhang, J. Wang, M. Chen, S. Li, Z. Lin, J. Li, Tumor-targeted aggregation of pH-sensitive nanocarriers for enhanced retention and rapid intracellular drug release. Polym. Chem. 5, 19 (2014)
S. Mura, J. Nicolas, P. Couvreur, Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 11 (2013)
J. Kim, J. Lee, Y.M. Lee, S. Pramanick, S. Im, W.J. Kim, Andrographolide-loaded polymerized phenylboronic acid nanoconstruct for stimuli-responsive chemotherapy. J. Control. Release. 259, 203–211 (2017)
H. Song, C. Wang, H. Zhang, L. Yao, J. Zhang, R. Gao, X. Tang, T. Chong, W. Liu, Y. Tang, A high-loading drug delivery system based on magnetic nanomaterials modified by hyperbranched phenylboronic acid for tumor-targeting treatment with pH response. Coll. Surf B.: Biointerfaces. 182, 110375 (2019)
G. Zheng, J. Zheng, L. Xiao, T. Shang, Y. Cai, Y. Li, Y. Xu, X. Chen, Y. Liu, B. Yang, Construction of a phenylboronic acid-functionalized nano-prodrug for pH-responsive emodin delivery and antibacterial activity. ACS Omega 6, 12 (2021)
J. Yan, G. Springsteen, S. Deeter, B. Wang, The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—it is not as simple as it appears. Tetrahedron 60, 49 (2004)
P. Zhang, Q. Xu, X. Li, Y. Wang, pH-responsive polydopamine nanoparticles for photothermally promoted gene delivery. Mater. Sci. Eng.: C. 108, 110396 (2020)
L. Zhu, Z. Song, S. Feng, H. Xu, S. Chen, R. Feng, Biotin-modified oligochitosan-F127 micelles for honokiol’s encapsulation. J. Nanopart. Res. 23, 5 (2021)
R. Feng, W. Wang, L. Zhu, H. Xu, S. Chen, Z. Song, Phenylboronic acid-functionalized F127-oligochitosan conjugate micelles for doxorubicin encapsulation. J. Biomed. Mater. Res. Part B. : Appl. Biomater. 108(8), 3345–3355 (2020)
S. Khoee, A. Kavand, A new procedure for preparation of polyethylene glycol-grafted magnetic iron oxide nanoparticles. J. Nanostruct. Chem. 4, 3 (2014)
A.A. D’souza, R. Shegokar, Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 13, 9 (2016)
L.E. Scheeren, D.R. Nogueira, L.B. Macedo, M.P. Vinardell, M. Mitjans, M.R. Infante, C.M. Rolim, PEGylated and poloxamer-modified chitosan nanoparticles incorporating a lysine-based surfactant for pH-triggered doxorubicin release. Coll. Surf B. Biointerfaces. 138, 117–127 (2016)
Y. Zhang, L. Tang, L. Sun, J. Bao, C. Song, L. Huang, K. Liu, Y. Tian, G. Tian, Z. Li, H. Sun, L. Mei, A novel paclitaxel-loaded poly(epsilon-caprolactone)/poloxamer 188 blend nanoparticle overcoming multidrug resistance for cancer treatment. Acta. Biomater. 6, 6 (2010)
C. Deng, C. Xu, X. Zhang, J.U. Yao, Y. Zhang, B.O. Yu, R.J. Lee, C. Jiang, A novel paclitaxel-loaded polymeric micelle system with favorable biocompatibility and superior antitumor activity. Anticancer. Res. 38, 1 (2018)
Y. Javadzadeh, F. Ahadi, S. Davaran, G. Mohammadi, A. Sabzevari, K. Adibkia, Preparation and physicochemical characterization of naproxen-PLGA nanoparticles. Coll. Surf B. Biointerfaces. 81, 2 (2010)
R. Savić, A. Eisenberg, D. Maysinger, Block copolymer micelles as delivery vehicles of hydrophobic drugs: Micelle–cell interactions. J. Drug. Target. 14, 6 (2006)
H.T. Chen, M.F. Neerman, A.R. Parrish, E.E. Simanek, Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J. Am. Chem. Soc. 126, 32 (2004)
Y. Liu, S. Fu, L. Lin, Y. Cao, X. Xie, H. Yu, M. Chen, H. Li, Redox-sensitive pluronic F127-tocopherol micelles: synthesis, characterization, and cytotoxicity evaluation. Int. J. Nanomed. 12, 2635-2644 (2017)
T. Ramasamy, Z.S. Haidar, T.H. Tran, J.Y. Choi, J.H. Jeong, B.S. Shin, H.G. Choi, C.S. Yong, J.O. Kim, Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta. Biomater. 10, 12 (2014)
S. Bhattacharjee, DLS and zeta potential—what they are and what they are not? J. Controll. Release.: Off. J. Controll. Release. Soc. 235, 337–351 (2016)
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31971301, 32171324), Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0149), and Fundamental Research Funds for Central Universities (2020CDJQY-A061, 2018CDHB1B08). In addition, some large instruments and equipment were provided by the Analysis and Testing Center of Chongqing University.
Author information
Authors and Affiliations
Contributions
WW and JL did the conception and design of the work. LZ did the acquisition and analysis of the data. SW interpreted the data. JL and LZ drafted the manuscript. YC and GW substantively revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Zhu, L., Wu, S. et al. MPEG-phenylboronic acid modified doxorubicin as the efficient pathological pH-responsive nanoplatform for potential anti-cancer delivery. Macromol. Res. 31, 181–191 (2023). https://doi.org/10.1007/s13233-022-00106-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-022-00106-5