Abstract
Purpose
Microbial exopolysaccharides (EPSs) are very important because they are used in biotechnological applications in different industrial areas. The aim of the study was to determine the best EPS producer Pleurotus sp., to optimize EPS production and to perform partial purification and characterization of the produced EPS.
Methods
After the production conditions were optimized, the EPS was isolated and partially purified. EPS was characterized by HP-TLC, 1H-NMR, FT-IR, and TGA. Hydroxyl, superoxide, and DPPH radical scavenging activities of the EPS were also investigated spectrophotometrically.
Result
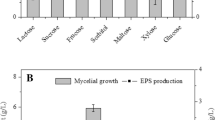
The best EPS producer and its incubation period in submerged fermentation were determined as Pleurotus sajor caju and on 5 days, respectively. Culture conditions to increase EPS production were optimized as follows (in per liter): 90 g of glucose, 10 g of yeast extract, 10 g of peptone, and 100 mM of Mg2+. The optimal initial pH, temperature, and an agitation rate of culture were determined as 5.0, 25 °C, and 150 rate min−1, respectively. The highest EPS production was determined as 33.32 ± 1.6 g L−1. After isolation of EPS, one active fraction was obtained by gel filtration chromatography. EPS is composed mainly of glucose according to HP-TLC analysis.
Conclusion
To the results, EPS had a complex structure by having carbohydrate and protein contents. The produced EPS had high degradation temperature as well as high antioxidant activity.








Similar content being viewed by others
References
Akpinar M, Ozturk Urek R (2017) Induction of fungal laccase production under solid state bioprocessing of new agroindustrial waste and its application on dye decolorization. 3 Biotech 7(98):1–10
Antón J, Meseguer I, Rodriguez Valera F (1988) Production of an extracellular polysaccharide by Haloferax mediterranei. Appl Environ Microbiol 54:2381–2386
Bayer AS, Eftekhar F, Tu J, Nast CC, Speert DP (1990) Oxygen-dependent up-regulation of mucoid exopolysaccharide (alginate) production in Pseudomonas aeruginosa. Infect Immun 58:1344–1349
Bazalel L, Hadar Y, Cerniglia C (1997) Enzymatic mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol 63:2495–2501
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao F, Bourven I, Guibaud G, Rene ER, Lens PNL, Pechaud Y, van Hullebusch ED (2018) Alteration of the characteristics of extracellular polymeric substances (eps) extracted from the fungus Phanerochaete chrysosporium when exposed to subtoxic concentrations of nickel (II). Int Biodeterior Biodegrad 129:179–188
Chardonnet CO, Sams CE, Conway WS (1999) Calcium effect on the mycelial cell walls of Botrytis cinerea. Phytochem 52:967–973
Cheng KC, Demirci A, Catchmark JM (2011) Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol 92:29–44
Copikova J, Cerna M, Novotna M, Kaasova JI, Synytsya A (2001) Application of FT-IR spectroscopy in detection of food hydrocolloids confectionery jellies and in food supplements. Czech J Food Sci 19:51–56
Dassy B, Stringfellow WT, Lieb M, Fournier JM (1991) Production of type 5 capsular polysaccharide by Staphylococcus aureus grown in a semi-synthetic medium. Microbiol 137:1155–1162
Decker EA, Welch B (1990) Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem 38:674–677
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Farres J, Caminal G, Lopez-Santin J (1997) Influence of phosphate on rhamnose-containing exopolysaccharide rheology and production by Klebsiella I-714. Appl Microbiol Biotechnol 48:522–527
Feng YL, Li WQ, Wu XQ, Cheng JW, Ma SY (2010) Statistical optimization of media for mycelial growth and exo-polysaccharide production by Lentinus edodes and a kinetic model study of two growth morphologies. Biochem Eng J 49:104–112
Friedemann TE, Haugen GE (1943) Pyruvic acid II. The determination of keto acids in blood and urine. J Biol Chem 147:415–442
Gao C, Wang Y, Wang C, Wang Z (2013) Antioxidant and immunological activity in vitro of polysaccharides from Gomphidius rutilus mycelium. Carbohydr Polym 92:2187–2192
Gern RMM, Wisbeck E, Rampinelli JR, Ninow JL, Furlan SA (2008) Alternative medium for production of Pleurotus ostreatus biomass and potential antitumor polysaccharides. Bioresour Technol 99:76–82
Görke B, Stülke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624
Halliwell B, Gutteridge JM, Aruoma OI (1987) The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165:215–219
Han SB, Lee CW, Kang JS, Yoon YD, Lee KH, Lee K, Park SK, Kim HM (2006) Acidic polysaccharide from Phellinus linteus inhibits melanoma cell metastasis by blocking cell adhesion and invasion. Int Immunopharmacol 6:697–702
He P, Geng L, Wang Z, Mao D, Wang J, Xu C (2012) Fermentation optimization, characterization and bioactivity of exopolysaccharides from Funalia trogii. Carbohydr Polym 89:17–23
Healy MG, Devine CM, Murphy R (1996) Microbial production of biosurfactants. Resour Conserv Recycl 18:41–57
Hwang HJ, Kim SW, Xu CP, Choi JW, Yun JW (2003) Production and molecular characteristics of four groups of exopolysaccharides from submerged culture of Phellinus gilvus. J Appl Microbiol 94:708–719
Ismail B, Nampoothiri KM (2010) Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch Microbiol 192:1049–1057
Jing X, Mao D, Geng L, Xu C (2013) Medium optimization, molecular characterization, and bioactivity of exopolysaccharides from Pleurotus eryngii. Arch Microbiol 195:749–757
Kim HO, Lim JM, Joo JH, Kim SW, Hwang HJ, Choi JW (2005) Optimization of submerged culture condition for the production of mycelial biomass and exopolysaccharides by Agrocybe cylindracea. Bioresour Technol 96:1175–1182
Kumar CG, Joo HS, Choi JW, Koo YM, Chang CS (2004) Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzym Microb Technol 34:673–681
Kumaran A (2006) Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem 97:109–114
Leong LP, Shui G (2002) An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem 76:69–75
Li R, Chen WC, Wang WP, Tian WY, Zhang XG (2010) Antioxidant activity of Astragalus polysaccharides and antitumour activity of the polysaccharides and siRNA. Carbohydr Polym 82:240–244
Li JE, Nie SP, Xie MY, Huang DF, Wang YT, Li C (2013) Chemical composition and antioxidant activities in immunosuppressed mice of polysaccharides isolated from Mosla chinensis Maxim cv. Jiangxiangru. Int Immunopharmacol 17:267–274
Lin ES, Chen YH (2007) Factors affecting mycelial biomass and exopolysaccharide production in submerged cultivation of Antrodia cinnamomea using complex media. Bioresour Technol 98:2511–2517
Lin R, Liu H, Wu S, Pang L, Jia M, Fan K, Jia S, Jia L (2012) Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int J Biol Macromol 51:153–157
Liu G, Miao X (2017) Switching cultivation for enhancing biomass and lipid production with extracellular polymeric substance as co-products in Heynigia riparia SX01. Bioresour Technol 207:214–220
Liu F, Ooi VE, Chang ST (1997) Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci 60:763–771
Ma L, Chen H, Zhu W, Wang Z (2013) Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Res Int 50:633–640
Mahapatra S, Banerjee D (2013) Fungal exopolysaccharide: production, composition and applications. Microbiol Insights 6:1–16
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Nakiboglu M, Ozturk Urek R, Ayar Kayali H, Tarhan L (2007) Antioxidant capacities of endemic Sideritis sipylea and Origanum sipyleum from Turkey. Food Chem 104:630–635
Nehad EA, El-Shamy AR (2010) Physiological studies on the production of exopolysaccharide by fungi. Agric Biol J N Am 1:1303–1308
Oh JY, Cho EJ, Nam SH, Choi JW, Yun JW (2007) Production of polysaccharide–peptide complexes by submerged mycelial culture of an entomopathogenic fungus Cordyceps sphecocephala. Process Biochem 42:352–362
Ooi VE, Liu F (2000) Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem 7:715–729
Osińska-Jaroszuk M, Jarosz-Wilkołazka A, Jaroszuk-Ściseł J, Szałapata K, Nowak A, Jaszek M, Ozimek E, Majewska M (2015) Extracellular polysaccharides from ascomycota and basidiomycota: production conditions, biochemical characteristics, and biological properties. World J Microbiol Biotechnol 31:1823–1844
Oyaizu M (1986) Antioxidative activities of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Raposo MFJ, Morais AMMB, Morais RMSC (2014) Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci 101:56–63
Ruas-Madiedo P, Hugenholtz J, Zoon P (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J 12:163–171
Shen JW, Shi CW, Xu CP (2013) Exopolysaccharides from Pleurotus pulmonarius: fermentation optimization, characterization and antioxidant activity. Food Technol Biotechnol 51:520–527
Silveira ML, Smiderle FR, Agostini F, Pereira EM, Bonatti-Chaves M, Wisbeck E, Ruthes AC, Sassaki GL, Cibriani TR, Furlan SA, Iacomini M (2015) Exopolysaccharide produced by Pleurotus sajor-caju: its chemical structure and anti-inflammatory activity. Int J Biol Macromol 75:90–96
Song TY, Lin HC, Yang NC, Hu ML (2008) Antiproliferative and antimetastatic effects of the ethanolic extract of Phellinus igniarius (Linnearus: Fries) Quelet. J Ethnopharmacol 115:50–56
Staub AM (1965) Removal of protein – Sevag method. Methods Carbohydr Chem 5:5–6
Tsiapali E, Whaley S, Kalbfleisch J, Ensley HE, Browder IW, Williams DL (2001) Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic Biol Med 30:393–402
Wang D, Sakoda A, Suzuki M (2001) Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour Technol 78:293–300
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974
Yang FC, Huang HC, Yang MJ (2003) The influence of environmental conditions on the mycelial growth of Antrodia cinnamomea in submerged cultures. Enzym Microb Technol 33:395–402
Yuan B, Chi X, Zhang R (2012) Optimization of exopolysaccharides production from a novel strain of Ganoderma lucidum CAU5501 in submerged culture. Braz J Microbiol 43:490–497
Zhang BB, Cheung PC (2011) Use of stimulatory agents to enhance the production of bioactive exopolysaccharide from Pleurotus tuber-regium by submerged fermentation. J Agric Food Chem 59:1210–1216
Zhao Q, Xie B, Yan J, Zhao F, Xiao J, Yao L, Zhao B, Huang Y (2012) In vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis. Carbohydr Polym 87:392–396
Zhen JQ, Wang JZ, Shi CW, Mao DB, He PX, Xu CP (2014) Characterization and antioxidant activity for exopolysaccharide from submerged culture of Boletus aereus. Process Biochem 49:1047–1053
Acknowledgments
This study was supported by the Scientific Research Project of Dokuz Eylül University (project number = 2014.KB.FEN.037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozturk Urek, R., Ilgin, S. Production and partial characterization of the exopolysaccharide from Pleurotus sajor caju. Ann Microbiol 69, 1201–1210 (2019). https://doi.org/10.1007/s13213-019-01502-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-019-01502-6