Abstract
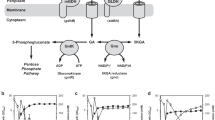
This study aimed to overexpress a glucose oxidase gene (GOD1) in Aureobasidium sp. P6 to achieve Ca2+-gluconic acid (GA) overproduction. The GOD1 gene was cloned, deleted, and overexpressed. A protein deduced from the GOD1 gene of Aureobasidium sp. P6 strain had 1824 bp that encoded a protein with 606 amino acids, with a conserved NADB-ROSSMAN domain and a GMC-oxred domain. Deleting the GOD1 gene made the disruptant GOK1 completely lose the ability to produce GA and GOD1 activity, whereas overexpressing the GOD1 gene rendered the transformant GOEX8 to produce considerably more Ca2+-GA (160.5 ± 5.6 g/L) and higher GOD1 activity (1438.6 ± 73.2 U/mg of protein) than its parent P6 strain (118.7 ± 4.3 g/L of Ca2+-GA and 1100.0 ± 23.6 U/mg of GOD1 protein). During a 10-L fermentation, the transformant GOEX8 grown in the medium containing 160.0 g/L of glucose produced 186.8 ± 6.0 g/L of Ca2+-GA, the yield was 1.2 g/g of glucose, and the volumetric productivity was 1.7 g/L/h. Most of the produced GOD1 were located in the yeast cell wall. The purified product was identified to be a GA. The transformant GOEX8 overexpressing the GOD1 gene could produce considerably more Ca2+-GA (186.8 ± 6.0 g/L) than its wild-type strain P6.





Similar content being viewed by others
References
Ahmed AS, Farag SS, Hassan IA, Botros HW (2015) Production of gluconic acid by using some irradiated microorganisms. J Radiat Res Appl Sci 8:374–380
Anastassiadis S, Rehm HJ (2006) Continuous gluconic acid production by Aureobasidium pullulans with and without biomass retention. Electron J Biotechnol 9(5):0–0
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–253
Canete-Rodríguez AM, Santos-Duenas IM, Jiménez-Hornero JE, Ehrenreich A, Liebl W, García-García I (2016) Gluconic acid: properties, production methods and applications—an excellent opportunity for agro-industrial by-products and waste bio-valorization. Process Biochem 5:1891–1903
Chi Z, Wang XX, Ma ZC, Buzdar MA, Chi ZM (2012) The unique role of siderophore in marine-derived Aureobasidium pullulans HN6.2. Biom 25:219–230
Courjean O, Mano N (2011) Recombinant glucose oxidase from Penicillium amagasakiense for efficient bioelectrochemical applications in physiological conditions. J Biotechnol 151:122–129
Forster A, Aurich A, Mauersberger S, Barth G (2007) Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl Microbiol Biotechnol 75:1409–1417
Fowler T, Rey MW, Vaha-Vahe P, Power SD, Berka RM (1993) The catR gene encoding a catalase from Aspergillus niger. Primary structure and elevated expression through increased copy number and use of a strong promoter. Mole Microbiol (5):989–998
Fu GY, Lu Y, Chi Z, Liu GL, Zhao SF, Jiang H, Chi ZM (2016) Cloning and characterization of a pyruvate carboxylase gene from Penicillium rubens and overexpression of the gene in the yeast Yarrowia lipolytica for enhanced citric acid production. Mar Biotechnol 18:1–14
Gao Z, Li Z, Zhang Y, Huang H, Li M, Zhou L, Tang Y, Yao B, Zhang W (2012) High-level expression of the Penicillium notatum glucose oxidase gene in Pichia pastoris using codon optimization. Biotechnol Lett 34:507–514
Green MR, Sambrook J, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th ed., cold Spring Harbor laboratory press, cold Spring Harbor, N.Y., pp:120–170
Guo Y, Lu F, Zhao H, Tang Y, Lu Z (2010) Cloning and heterologous expression of glucose oxidase gene from Aspergillus niger Z-25 in Pichia pastoris. Appl Biochem Biotechnol 162:498–509
Hatziuikolaou DG, Macris BJ (1995) Factors regulating production of glucose oxidase by Aspergillus niger. Enzy Microb Technol 17:530–534
Horaguchi Y, Saito S, Kojima K, Tsugawa W, Ferri S, Sode K (2012) Construction of mutant glucose oxidases with increased dye-mediated dehydrogenase activity. Intern J Mole Sci 13:14149–14157
Kona RP, Qureshi N, Pai JS (2001) Production of glucose oxidase using Aspergillus niger and corn steep liquor. Bioresour Technol 78:123–126
Kovacevic G, Blazic M, Draganic B, Ostafe R, Gavrovic-Jankulovic M, Fischer R, Prodanovic R (2014) Cloning, heterologous expression, purification and characterization of M12 mutant of Aspergillus niger glucose oxidase in yeast Pichia pastoris KM71H. Mol Biotechnol 56:305–311
Liu GL, Wang DS, Wang LF, Zhao SF, Chi ZM (2011) Mig1 is involved in mycelial formation and expression of the genes encoding extracellular enzymes in Saccharomycopsis fibuligera A11. Fung Genet Biol 48:904–913
Ma Y, Wang GY, Liu GL, Wang ZP, Chi ZM (2013) Overproduction of poly(β-malic acid) (PMA) from glucose by a novel Aureobasidium sp. P6 strain isolated from mangrove system. Appl Microbiol Biotechnol 97:8931–8939
Mischak H, Kubicek CP, Rohr R (1985) Formation and location of glucose oxidase in citric acid producing mycelia of Aspergillus niger. Appl Microbiol Biotechnol 21:27–31
Pal P, Kumar R, Banerjee S (2016) Manufacture of gluconic acid: a review towards process intensification for green production. Chem Eng Process 104:160–171
Ramachandran S, Fontanille P, Pandey A, Larroche C (2006) Gluconic acid: properties, applications and microbial production. Food Technol Biotechnol 44 (2):185–195
Ramachandran S, Nair S, Larroche C (2017) 26-Gluconic acid[M]//current developments in biotechnology and bioengineering. Elsevier:577–599
Rocha SN, Abrahão-Neto J, Cerdán ME, González-Siso MI, Gombert AK (2010) Heterologous expression of glucose oxidase in the yeast Kluyveromyces marxianus. Microb Cell Factories 9:4
Spiro RG (1966) Analysis of sugars found in glycoproteins. Meth Enzymol 8:3–26
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 24:4876–4882
Swart K, van de Vondervoort PJI, Witteveen CFB, Visser J (1990) Genetic localisation of a series of genes affecting glucose oxidase levels in Aspergillus niger. Current Genet 18:435–439
van Dijken JP, Veenhuis M (1980) Cytochemical localization of glucose oxidase in peroxisomes of Aspergillus niger. Euro J Appl Microbiol Biotechnol 9:275–283
Wang QQ, Lu Y, Ren ZY, Chi Z, Liu GL, Chi ZM (2017) CreA is directly involved in pullulan biosynthesis and regulation of Aureobasidium melanogenum P16. Curr Genet 63:471–485
Witteveen CFB, Veenhuis M, Visser J (1992) Localization of glucose oxidase and catalase activities in Aspergillus niger. Appl Environ Microbiol 58:1190–1194
Wong CM, Wong KH, Chen XD (2008) Glucose oxidase: natural occurrence, function, properties and industrial applications. Appl Microbiol Biotechnol 78:927–938
Yang L, Lübeck M, Lübeck PS (2014) Deletion of glucose oxidase changes the pattern of organic acid production in Aspergillus carbonarius. AMB Exp 4:54
Zhang F, Wang ZP, Chi Z, Raoufi Z, Abdollahi S, Chi ZM (2013) The changes in Tps1 activity, trehalose content and expression of TPS1 gene in the psychrotolerant yeast Guehomyces pullulans 17-1 grown at different temperatures. Extremophiles 17:241–249
Zhang FL, Chi ZM, Zhu KL, Li J, Li MJ, Liang LK, Wu LF (2007) Expression in Escherichia coli of the recombinant Vibrio anguillarum metalloprotease and its purification and characterization. World J Microbiol Biotechnol 23:331–337
Zhang H, Zhang J, Bao J (2016) High titer gluconic acid fermentation by Aspergillus niger from dry dilute acid pretreated corn stover without detoxification. Bioresour Technol 203:211–219
Funding
This research was supported by National Natural Science Foundation of China and the Grant No. is 31500029.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Supplementary File 1
Primers used in this study (DOC 32 kb)
Supplementary File 2
Construction of the vector for disruption of the GOD1 gene (DOC 509 kb)
Supplementary File 3
Construction of a vector for over-expression of the GOD1 gene (DOC 295 kb)
Supplementary File 4
Nucleotide sequence of the Aureobasidium sp. P6 strain GOD1 gene, upstream regions, and deduced amino acid sequence. The promoter is underlined, two spn-1 elements, one CAAT box, one TATA box are boxed, the transcriptional start site is indicated by a big arrow. The amino acids in shade are the GMC-oxred domain of the oxidoreductase family, the amino acids double-underlined are the FAD-binding site, the amino acids in both italics and bold are the signal peptide and the cleavage site is indicated by an arrow, N-glycosylation sites are shown by the oval frames (DOC 950 kb)
Supplementary File 5
Ca2+-GA production by different transformants and their parent strain P6. Data are given as mean ± SD, n = 3. ** (p < 0.01) extremely significant difference compared with that of Aureobasidium sp. P6 strain (DOC 371 kb)
Supplementary File 6
The ribbon representation of the secondary structure elements of the GOD1 from Aureobasidium sp. P6 strain, showing a homodimer (orange and blue) (color figure online) (DOC 184 kb)
Rights and permissions
About this article
Cite this article
Ma, Y., Chi, Z., Li, YF. et al. Cloning, deletion, and overexpression of a glucose oxidase gene in Aureobasidium sp. P6 for Ca2+-gluconic acid overproduction. Ann Microbiol 68, 871–879 (2018). https://doi.org/10.1007/s13213-018-1393-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-018-1393-4