Abstract
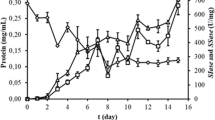
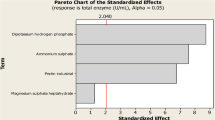
The aims of the this study are to select the best cultivation type for plant growth regulator (PGR) production, to optimize PGR production with statistical experimental design, and to calculate bioprocess parameters and yield factors during PGR production by P. eryngii in flask and reactor scales. Submerged fermentation was the best cultivation type with 4438.67 ± 37.14, 436.95 ± 27.31, and 54.32 ± 3.21 mg/L of GA3, ABA, and IAA production values, respectively. The Plackett–Burman and Box–Behnken designs were used to determine effective culture parameters and interactive effects of the selected culture parameters on PGR production by Pleurotus eryngii under submerged fermentation. The statistical model is valid for predicting PGR production by P. eryngii. After these studies, maximum PGR production (7926.17 ± 334.09, 634.92 ± 12.15, and 55.41 ± 4.38 mg/L for GA3, ABA, and IAA, respectively) was reached on the 18th day of fermentation under optimized conditions. The optimum formula was 50 g/L fructose, 3 g/L NaNO3, and 1.5 g/L KH2PO4, 1 mg/L thiamine, incubation temperature 25 °C, initial medium pH 7.0, and an agitation speed of 150 rpm. The kinetics of PGR production was investigated in batch cultivation under 3-L stirred tank reactor conditions. Concentrations of GA3, ABA, and IAA of 10,545.00 ± 527.25, 872.32 ± 21.81, and 60.48 ± 3.48 mg/L were obtained at the reactor scale which were 4.1, 3.4, and 2.3 times higher than the initial screening values. The specific growth rate (µ), the volumetric (rp) and specific (Qp) PGR production rates, 486.11 mg/L/day and 107.43 mg/g biomass/day for GA3, confirmed the successful transfer of optimized conditions to the reactor scale. In the presented study, PGR production of P. eryngii is reported for the first time.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Akyüz M, Kırbağ S, Karatepe M, Güvenç M, Zengin F (2011) Vitamin and fatty acid composition of P. eryngii var. eryngii. Bitlis Eren Univ J Sci Technol 1:16–20
Alam N, Yoon KN, Lee JS, Cho HJ, Shim MJ, Lee TS (2011) Dietary effect of Pleurotus eryngii on biochemical function and histology in hypercholesterolemic rats. Saudi J Biol Sci 18:403–409
Başıaçık Karakoç Ş, Aksöz N (2005) Bazı matrikslere tutuklanmiş Aspergillus niger’den gibberellik asit üretimi. Orlab on Line Mikrobiyol Derg 3:16 (In Turkish)
Bayburt C, Karaduman AB, Yenice Gürsu B, Tuncel M, Yamaç M (2020) Decolourization and detoxification of textile dyes by Lentinus arcularius in immersion bioreactor scale. Int J Environ Sci Technol 17:945–958
Bose A, Shah D, Keharia H (2013) Production of indole-3-acetic-acid (IAA) by the white rot fungus Pleurotus ostreatus under submerged condition of Jatropha seedcake. Mycology 4:103–111
Carlavilla JR, Manjón JL (2023) The king oyster mushroom Pleurotus eryngii behaves as a necrotrophic pathogen of Eryngium campestre. Ital J Mycol 52:22–31
Cen YK, Li MH, Wang Q, Zhang JM, Yuan JC, Wang YS, Liu ZQ, Zheng Y (2023) Evolutionary engineering of Fusarium fujikuroi for enhanced production of gibberellic acid. Process Biochem 125:7–14
Curtis PJ, Cross BE (1954) Gibberellic acid. A new metabolite from the culture filtrates of Gibberella fujikuroi. Chem Ind 35:1066
de Oliveira J, Rodrigues C, Vandenberghe LPS, Câmara MC, Libardi N, Soccol CR (2017) Gibberellic acid production by different fermentation systems using citric pulp as substrate/support. BioMed Res Int 2017:5191046
Doğan B, Yıldız Z, Aksöz N, Eninanç AB, Dağ İ, Yıldız A, Doğan HH, Yamaç M (2023) Flask and reactor scale production of plant growth regulators by Inonotus hispidus: optimization, immobilization and kinetic parameters. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2023.2185636. (In press)
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Durán-Páramo E, Molina-Jiménez H, Brito-Arias MA, Martínez FR (2004) Gibberellic acid production by free and immobilized cells in different culture systems. Appl Biochem Biotechnol 114:381–388
El-Sheikh MA, Rajaselvam J, Abdel-Salam EM, Vijayaraghavan P, Alatar AA, Biji GD (2020) Paecilomyces sp. ZB is a cell factory for the production of gibberellic acid using a cheap substrate in solid state fermentation. Saudi J Biol Sci 27:2431–2438
Epstein E, Miles PG (1967) Identification of indole-3-acetic acid in the basidiomycete Schizophyllum commune. Plant Physiol 42:911–914
Fushimi K, Anzai K, Tokuyama S, Kiriiwa Y, Matsumoto N, Sekiya A, Hashizume D, Nagasawa K, Hirai H, Kawagishi H (2012) Agrocybynes A–E from the culture broth of Agrocybe praecox. Tetrahedron 68:1262–1265
Gordon SA, Fleck A, Bell J (1978) Optimal conditions for the estimation of ammonium by the Berthelot reaction. Ann Clin Biochem 15:270–275
Gruen HE (1959) Auxins and Fungi. Ann Rev Plant Physiol 10:405–440
Hamayun M, Khan S, Khan MA, Khan AL, Kang SM, Kim SK, Joo GJ, Lee IJ (2009) Gibberellin production by pure cultures of a new strain of Aspergillus fumigatus. World J Microbiol Biotechnol 25:1785–1792
Hwang HJ, Kim SW, Xu CP, Choi JW, Yun JW (2004) Morphological and rheological properties of the three different species of basidiomycetes Phellinus in submerged cultures. J Appl Microbiol 96:1296–1305
Isa NKM, Don MM (2014) Investigation of the Gibberellic acid optimization with a statistical tool from Penicillium variable in batch reactor. Prep Biochem Biotechnol 44(6):572–585
Khan SA, Hamayun M, Kim HY, Yoon HJ, Lee IJ, Kim JG (2009) Gibberellin production and plant growth promotion by a newly isolated strain of Gliomastix murorum. World J Microbiol Biotechnol 25:829–833
Kobori H, Sekiya A, Suzuki T, Choi JH, Hirai H, Kawagishi H (2015) Bioactive sesquiterpene aryl esters from the culture broth of Armillaria sp. J Nat Prod 78:163–167
Lale GJ, Gadre RV (2016) Production of Bikaverin by a Fusarium fujikuroi mutant in submerged cultures. AMB Express 6(1):34
Liu X, Zhou B, Kin RS, Jia L, Deng P, Fan YKM (2010) Extraction and antioxidant activities of intracellular polysaccharide from Pleurotus sp. mycelium. Int J Biol Macromol 47:116–119
Machado CMM, Soccol CR, De Oliveira BH, Pandey A (2002) Gibberellic acid production by solid-state fermentation in coffee husk. Appl Biochem Biotechnol 102–103:179–191
Monrro M, Garcia JR (2022) Gibberellic acid production from corn cob residues via fermentation with Aspergillus niger. J Chem 2022:1112941
Pham MT, Huang CM, Kirschner R (2019) The plant growth-promoting potential of the mesophilic wood-rot mushroom Pleurotus pulmonarius. J Appl Microbiol 127:1157–1171
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305–325
Rangaswamy V (2012) Improved production of gibberellic acid by Fusarium moniliforme. J Microbiol Res 2(3):51–55
Seyis Bilkay I, Karakoç Ş, Aksöz N (2010) Indole-3-acetic acid and gibberellic acid production in Aspergillus niger. Turk J Biol 34:313–318
Shenbhgaraman R, Jagadish LK, Premalatha K, Kaviyarasan V (2012) Optimization of extracellular glucan production from Pleurotus eryngii and its impaction angiogenesis. Int J Biol Macromol 50:957–964
Shukla R, Chand S, Srivastava AK (2005) Batch kinetics and modeling of gibberellic acid production by Gibberella fujikuro. Enzyme Microb Technol 36:492–497
Silva EME, Dendooven L, Reynell JAU, Ramirez AIM, Gonzalez Alatorre G, Martinez MT (1999) Morphological development and gibberellin production by different strains of Gibberella fujikuroi in shake flasks and bioreacto. World J Microbiol Biotechnol 15:753–755
Silva EME, Dendooven L, Magana IP, Parra RP, de la Torre M (2000) Optimization of gibberellic acid production by immobilized Gibberella fujikuroi mycelium in fluidized bioreactors. J Biotechnol 76:147–155
Stajić M, Vukojević J, Duletić-Laušević S (2009) Biology of Pleurotus eryngii and role in biotechnological processes: a review. Crit Rev Biotechnol 29(1):55–66
Swain MR, Ray RC (2008) Optimization of cultural conditions and their statistical interperation for production of indole-3-acetic acid by Bacillus subtilis CM5 using cassava fibrous residue. J Sci Ind Res 67:622–628
Takayama T, Yoshida H, Araki K, Nakayama K (1983) Microbial production of abscisic acid with Cercospora rosicola. Biotechnol Lett 5:55–58
Tiryaki D, Gülmez Ö (2021) Determination of the effect of indole acetic acid (IAA) produced from edible mushrooms on plant growth and development. Anatol J Biol 2:17–20
Ünyayar S, Topcuoglu SF, Ünyayar A (1996) A modified method for extraction and identification of indole-3-acetic acid (IAA), gibberellic acid (GA3), abscisic acid (ABA) and zeatin produced by Phanerochaete chrysosporium ME 446 Bulgar. J Plant Physiol 22:105–110
Ünyayar S, Ünyayar A, Ünal E (2000) Production of auxin and abscisic acid by Phanerochaete chrysosporium ME446 immobilized on polyurethane foam. Turk J Biol 24:769–774
Valentino MJG, Galvez CT (2015) Auxin-like and gibberellic acid-like activity of Pleurotus sajor-caju (Fr.) Singer and Volvariella volvacea Fr. on Tomato (Lycopersicon esculentum Mill.) seedlings. Adv Environ Biol 9(23):361–367
Wang B, Yin K, Wu C, Wang L, Yin L, Lin H (2022) Medium optimization for GA4 production by Gibberella fujikuroi using response surface methodology. Fermentation 8:230
Wei W, Hui WY, Lie LJ, Fei YY (2019) Enhancement of gibberellin acid production through pH regulation in batch fermentation of Gibberella fujikuroi. Mycosystema 38(7):1185–1190
Werle LB, Abaide ER, Felin TH, Kuhn KR, Tres MV, Zabot GL, Kuhn RC, Jahn SL, Mazutti MA (2020) Gibberellic acid production from Gibberella fujikuroi using agro-industrial residues. Biocat Agric Biotechnol 25:101608
Wu J, Kobori H, Kawaide M, Suzuki T, Choi JH, Yasuda N, Noguchi K, Matsumoto T, Hirai H, Kawagishi H (2013) Isolation of bioactive steroids from the Stropharia rugosoannulata mushroom and absolute configuration of Strophasterol B. Biosci Biotechnol Biochem 77:1779–1781
Wu J, Uchida K, Ridwan AY, Kondo M, Choi JH, Hirai H, Kawagishi H (2019) Erinachromanes A and B and Erinaphenol A from the culture broth of Hericium erinaceus. J Agric Food Chem 67:3134–3139
Yaoita Y, Yoshihara Y, Kakuda R, Machida K, Kikuchi M (2002) New sterols from two edible mushrooms Pleurotus eryngii and Panellus serotinus. Chem Pharmac Bull 50:551–553
Yürekli F, Yesilada Ö, Yürekli M, Topcuoglu SF (1999) Plant growth hormone production from olive oil mill and alcohol factory wastewaters by white rot fungi. World J Microbiol Biotechnol 15:503–505
Yürekli F, Gecgil H, Topcuoglu SF (2003) The synthesis of indole-3-acetic acid by the industrially important white-rot fungus Lentinus sajor-caju under different culture conditions. Mycol Res 107:305–309
Zervakis GI, Venturella G, Papadopoulou K (2001) Genetic polymorphism and taxonomic infrastructure of the Pleurotus eryngii species-complex as determined by RAPD analysis, isozyme profiles and ecomorphological characters. Microbiology 147(11):3183–3194
Zhang B, Lei Z, Liu Z-Q, Zheng Y-G (2020) Improvement of gibberellin production by a newly isolated Fusarium fujikuroi mutant. J Appl Microbiol 129(6):1620–1632
Acknowledgements
The authors would like to thank Dr. Abdunnasır Yıldız who donated the studied isolate and to the Eskişehir Osmangazi University Research Fund (201219A106) for financing this study.
Author information
Authors and Affiliations
Contributions
MY designed the experiment with the help of NA. BD, ABE, and BGKK performed the study and ZY analyzed the data. MY, NA, and ZY finalized the manuscript before final submission.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Research involving human participants and/or animals
The authors declare that human participants or animals were not included in this study.
Informed consent
The authors declared that this research does not require informed consent since it is not a clinical trial.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doğan, B., Yıldız, Z., Aksöz, N. et al. Optimization and reactor-scale production of plant growth regulators by Pleurotus eryngii. 3 Biotech 13, 314 (2023). https://doi.org/10.1007/s13205-023-03744-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03744-3