Abstract
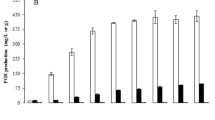
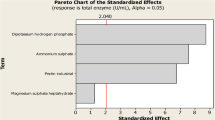
Alpha keto acids are deaminated forms of amino acids that have received significant attention as feed and food additives in the agriculture and medical industries. To date, their production has been commonly performed at shake-flask scale with low product concentrations. In this study, production of phenylpyruvic acid (PPA), which is the alpha keto acid of phenylalanine was investigated. First, various microorganisms were screened to select the most efficient producer. Thereafter, growth parameters (temperature, pH, and aeration) were optimized in bench scale bioreactors to maximize both PPA and biomass concentration in bench scale bioreactors, using response surface methodology. Among the four different microorganisms evaluated, Proteus vulgaris was the most productive strain for PPA production. Optimum temperature, pH, and aeration conditions were determined as 34.5 °C, 5.12, and 0.5 vvm for PPA production, whereas 36.9 °C, pH 6.87, and 0.96 vvm for the biomass production. Under these optimum conditions, PPA concentration was enhanced to 1,054 mg/L, which was almost three times higher than shake-flask fermentation concentrations. Moreover, P. vulgaris biomass was produced at 3.25 g/L under optimum conditions. Overall, this study demonstrated that optimization of growth parameters improved PPA production in 1-L working volume bench-scale bioreactors compared to previous studies in the literature and was a first step to scale up the production to industrial production.








Similar content being viewed by others
Abbreviations
- PPA:
-
Phenylpyruvic acid
- RSM:
-
Response surface methodology
- TSB:
-
Trypticase soy broth
- DNPH 2:
-
4-Dinitrophenylhydrazine
- HPLC:
-
High pressure liquid chromatography
- PTFE:
-
Polytetrafluoroethylene
- OPA:
-
o-Phthalaldehyde
- ANOVA:
-
Analysis of variation
References
Masato T, Toshiaki K, Toshiyasu S (1986) Process of producing alpha-keto acids. Letters Patent of the United States, vol 17, pp 1385–1394
Pailla K, Cynober FB, Aussel C, De Bandt JP, Cynober L (2000) Branched-chain keto-acids and pyruvate in blood: measurement by HPLC with fluorimetric detection and changes in older subjects. Clin Chem 46:848–853
Folling I (1994) The discovery of phenylketonuria. Acta Paediatr Suppl 407:4–10
Krause FS, Blombach B, Eikmanns BJ (2010) Metabolic engineering of Corynebacterium glutamicum for 2-ketoisovalerate production. Appl Environ Microb 76:8053–8061
Casey MG, Bosset BJ, Butikofer U, Wyder MTF (2004) Effect of alpha keto acids on the development of flavour in Swiss Gruyere type cheese. Lebensm-Wiss u.-Technol 37:269–273
Gaby AL, Chawla RK (1976) Efficiency of phenylpyruvic and phenyllactic acids as substitutes for phenylalanine in the diet of the growing rat. J Nutr 106:158–168
Summer JD (1993) Reducing nitrogen excretion of the laying hem by feeding lower crude protein diets. Poult Sci 72:1473–1478
Smit JA (1966) Specific activity of phenylalanine deaminase in extracts of the Proteus-Providence group. Nature 211:1003
Takagi Y, Sugisawa T, Hoshino T (2009) Continuous 2-Keto-l-gulonic acid fermentation by mixed culture of Ketogulonicigenium vulgare DSM 4025 and Bacillus megaterium or Xanthomonas maltophilia. Appl Microbiol Biotechnol 86:469–480
Chernyavskaya OG, Shishkanov NV, Il’chenko AP, Finogenova TV (2000) Synthesis of α-ketoglutaric acid by Yarrowia lipolytica yeast grown on ethanol. Appl Microbiol Biotechnol 53:152–158
Sluis CVD, Rahardjo YSP, Smit BA, Kroon PJ, Hartmans S, Schure EGT, Tramper J, Wijffels RH (2001) Contaminant extracellular accumulation of alpha keto acids and higher alcohols by Zygosaccharomyces rouxii. J Biosci Bioeng 93:117–124
Itoh U, Tsuchiya H, Sato M, Namikawa I (1994) Characterization of oral Eubacterium species by α-keto acid production from amino acids. Lett Appl Microbiol 19:261–264
Tsuchiya H, Sato M, Yamamato K, Yamauchi M, Tani H, Namikawa I, Takagi N (1990) High-performance liquid chromatographic analysis of α-keto acids produced from amino acid metabolism in oral. Bacteroides. J Appl Bacteriol 69:125–133
Shuler ML, Kargi F (1992) Bioprocess engineering basic concepts. Prentice Hall, Englewood Cliffs 07632
Ederer GM, Chu JH, Blazevic DJ (1971) Rapid test for urease and phenylalanine deaminase production. App Microbiol 21:545
Elias RJ, Laurieb VF, Ebelera SE, Wongc JW, Waterhouse AL (2008) Analysis of selected carbonyl oxidation products in wine by liquid chromatography with diode detection. Anal Chim Acta 626:104–110
Lafuente RF, Rodriguez V, Guisan JM (1988) The coimmobilization of d-amino acid oxidase and catalase enables the quantitative transformation of D-amino acids (d-phenylalanine) into α-keto acids (phenylpyruvic acid). Enzyme Microb Tech 23:28–33
Xu P, Qui J, Gao C, Ma C (2008) Biorechnological routes to pyruvate production. J Biosci Bioeng 205:169–175
Takahashi E, Ito K, Yoshimoto T (1999) Cloning of L-amino acid deaminase gene from Proteus vulgaris. Biosci Biotechnol Biochem 63:2244–2247
Ware GC (1954) The effect of incubation temperature on the growth: requirements of Proteus vulgaris and Salmonella typhi. J Gen Microbiol 11:398–400
Szwajcer E, Brodelius P, Mosbach K (1982) Production of α-keto acids: 2. Immobilized whole cells of Providencia sp. PCM 1298 containing L-amino acid oxidase. Enzyme Microb Technol 4:409–413
Sultana RS, Arifin BMS, Islam MM, Kumar A (2010) Demonstration of mass transfer using aeration of water. J Chem Eng 25:56–60
Acknowledgments
This work was supported in part by Turkish Ministry of Education by providing scholarship to Hasan Bugra Coban and the Pennsylvania Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coban, H.B., Demirci, A., Patterson, P.H. et al. Screening of phenylpyruvic acid producers and optimization of culture conditions in bench scale bioreactors. Bioprocess Biosyst Eng 37, 2343–2352 (2014). https://doi.org/10.1007/s00449-014-1212-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1212-7