Abstract
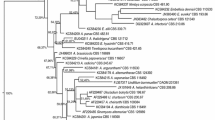
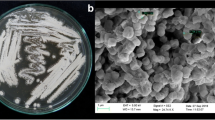
The purpose of the present study was to discover antimicrobial endophytic fungi from Astragalus chinensis. Three fungal endophytes with antibacterial activity were isolated and determined as Chaetomium sp. HQ-1, Fusarium sp. HQ-7 and Fusarium sp. HQ-9 based on the neighbor-joining phylogenetic tree. Chaetomium sp. HQ-1 showed the best antibiotic potential and was thus selected for large-scale fermentation. Bioactivity-directed separation of ME fermentation of strain HQ-1 led to the discovery of three compounds, which were identified as differanisole A (1), 2,6-dichloro-4-propylphenol (2) and 4,5-dimethylresorcinol (3), from the HR–ESI–MS and NMR data analysis. All three compounds exhibited moderate antibacterial activity against Listeria monocytogenes, Staphylococcus aureus, and methicillin-resistant S. aureus, with MIC values ranging from 16 to 128 μg/mL. Compounds 1 and 3 also displayed promising antifungal activity against Selerotium rolfsii with IC50 values of less than 16 and 32 μg/mL, respectively, which were comparable to that of actidione (8 μg/mL). The findings of the present study suggest that the endophytic fungi from A. chinensis have the potential to be used as bactericides and fungicides.



Similar content being viewed by others
References
Alvin A, Miller KI, Neilan BA (2014) Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol Res 169:483–495
Asgari B, Zare R (2011) The genus Chaetomium in Iran, a phylogenetic study including six new species. Mycologia 103(4):863–882
Basak S, Singh P, Rajurkar M (2016) Multidrug resistant and extensively drug resistant bacteria: a study. J Pathog. https://doi.org/10.1155/2016/4065603
Bashyal BP, Wijeratne EMK, Tillotson J, Arnold AE, Chapman E, Gunatilaka AAL (2017) Chlorinated dehydrocurvularins and alterperylenepoxide A from Alternaria sp. AST0039, a fungal endophyte of Astragalus lentiginosus. J Nat Prod 80:427–433
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the infectious diseases society of America. Clin Infect Dis 48:1–12
Christie RM, Rickards RW, Schmalzl KJ, Taylor D (1977) Ring contraction of 4-substituted 2, 6-dichlorophenols. The crystal structure of 2, 2, 4α, 5α-tetrachloro-1α, 3α-dihydroxycyclopentane-1, 4-carbolactone. Aust J Chem 30(10):2195–2204
Egamberdieva E, Wirth S, Behrendt U, Ahmad P, Berg G (2017) Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front Microbiol 8:199
Fan YP, Hu YL, Wang DY, Liu JG, Zhang J, Zhao XJ, Liu X, Liu C, Yuan J, Ruan S (2012) Effects of Astragalus polysaccharide liposome on lymphocyte proliferation in vitro and adjuvanticity in vivo. Carbohydr Polym 88:68–74
Golinska P, Wypij M, Agarkar G, Rathod D, Dahm H, Rai M (2015) Endophytic actinobacteria of medicinal plants: diversity and bioactivity. Anton Leeuw 108:267–289
Gouda S, Das G, Sen SK, Shin HS, Patra JK (2016) Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol 7:1538
Hamon M, Bierne H, Cossart P (2006) Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 4:423–434
Hoffmann S, Batz MB, Morris JG Jr (2012) Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot 75:1292–1302
Ibrahim LF, Marzouk MM, Hussein SR, Kawashty SA, Mahmoud K, Saleh NAM (2013) Flavonoid constituents and biological screening of Astragalus bombycinus. Nat Prod Res 27:386–393
Jiang JB, Wu CH, Gao H, Song JD, Li HQ (2010) Effects of astragalus polysaccharides on immunologic function of erythrocyte in chickens infected with infectious bursa disease virus. Vaccine 28:5614–5616
Jogi A, Kerry JW, Brenneman TB, Leebens-Mack JH, Gold SE (2016) Identification of genes differentially expressed during early interactions between the stem rot fungus (Sclerotium rolfsii) and peanut (Arachis hypogaea) cultivars with increasing disease resistance levels. Microbiol Res 184:1–12
Kanatani Y, Makishima M, Ken-i Asahi, Sakurai A, Takahashi N, Motoyoshi K, Nagata N (1997) Differanisole A, a novel antitumor antibiotic, enhances growth inhibition and differentiation of human myeloid leukemia cells induced by 9-cis retinoic acid. BBA Mol Cell Res 1359:71–79
Kubohara Y, Okamoto K, Tanaka Y, Ken-i Asahi, Sakurai A, Takahashi N (1993) Differanisole A, an inducer of the differentiation of Friend leukemic cells, induces stalk cell differentiation in Dictyostelium discoideum. FEBS Lett 322(1):73–75
Lewis K (2012) Antibiotics: recover the lost art of drug discovery. Nature 485:439–440
Li R, Chen WC, Wang WP, Tian WY, Zhang XG (2010) Antioxidant activity of Astragalus polysaccharides and antitumour activity of the polysaccharides and siRNA. Carbohydr Polym 82(2):240–244
Li X, Qu L, Dong Y, Han L, Liu E, Fang S, Zhang Y, Wang T (2014) A review of recent research progress on the Astragalus genus. Molecules 19(11):18850–18880
Li H, Tian JM, Tang HY, Pan SY, Zhang AL, Gao JM (2015) Chaetosemins A–E, new chromones isolated from an Ascomycete Chaetomium seminudum and their biological activities. RSC Adv 5:29185–29192
Liu X, Li H, Zhou F, Wang R (2015) Secondary metabolites of Fusarium sp., an endophytic fungus in Astragalus membranaceus. Chem Nat Compd 51(6):1199–1201
Liu P, Zhao H, Luo Y (2017) Anti-aging implications of Astragalus membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis 8(6):868–886
Liu D, Chen L, Zhao J, Cui K (2018) Cardioprotection activity and mechanism of Astragalus polysaccharide in vivo and in vitro. Int J Biol Macromol 111:947–952
Martinez-Klimova E, Rodríguez-Peña K, Sánchez S (2017) Endophytes as sources of antibiotics. Biochem Pharmacol 134:1–17
Mazinani Z, Zamani M, Sardari S (2017) Isolation and identification of phyllospheric bacteria possessing antimicrobial activity from Astragalus obtusifolius, Prosopis juliflora, Xanthium strumarium and Hippocrepis unisiliqousa. Avicen J Med Biotechnol 9(1):31–37
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3):629–661
Ogata N, Shibata T (2004) Inhibition of rat intestinal Cl− secretion by 4,5-dimethylresorcinol. Pharmacology 72(4):247–253
Oka H, Asahi KI, Morishima H, Sanada M, Shiratori K, Iimura Y, Sakurai T, Uzawa J, Iwadare S, Takahashi N (1985) Differanisole A, a new differentiation inducing substance. J Antibiot 38(8):1100–1102
Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM (2011) Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15
Schulz B, Wanke U, Draeger S, Aust HJ (1993) Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res 97:1447–1450
Sharma D, Pramanik A, Agrawal PK (2016) Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D.Don. 3 Biotech 6(210):1–14
Spellberg B (2012) New antibiotic development: barriers and opportunities in 2012. APUA Clin Newsl 30:8–10
Strobel GA (2003) Endophytic as sources of bioactive products. Microbes Infect 5:535–544
Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18:448–459
Tian Y, Jiang N, Zhang AH, Chen CJ, Deng XZ, Zhang WJ, Tan RX (2015) Muta-mycosynthesis of naphthalene analogs. Org Lett 17:1457–1460
Visser AA, Nobre T, Currie CR, Aanen DK, Poulsen M (2012) Exploring the potential for Actinobacteria as defensive symbionts in fungus-growing termites. Microb Ecol 63:975–985
Vuong C, Yeh AJ, Cheung GYC, Otto M (2016) Investigational drugs to treat methicillin-resistant Staphylococcus aureus. Expert Opin Investig Drug 25(1):73–93
Willyard C (2017) The drug-resistant bacteria that pose the greatest health threats. Nat New. https://doi.org/10.1038/nature.2017.21550
Wu CY, Ke Y, Zeng YF, Zhang YW, Yu HJ (2017) Anticancer activity of Astragalus polysaccharide in human non-small cell lung cancer cells. Cancer Cell Int 17:115
Xu GB, Li LM, Yang T, Zhang GL, Li GY (2012) Chaetoconvosins A and B, alkaloids with new skeleton from fungus Chaetomium convolutum. Org Lett 14:6052–6055
Xu YM, Artiles PE, Liu MX, Arnold AE, Gunatilaka AAL (2013) Secoemestrin D, a cytotoxic epitetrathiodioxopiperizine, and emericellenes A–E, five sesterterpenoids from Emericella sp. AST0036, a fungal endophyte of Astragalus lentiginosus. J Nat Prod 76:2330–2336
Yan W, Ge HM, Wang G, Jiang N, Mei YN, Jiang R, Li SJ, Chen CJ, Jiao RH, Xu Q, Ng SW, Tan RX (2014) Pictet-Spengler reaction-based biosynthetic machinery in fungi. Proc Natl Acad Sci 111:18138–18143
Yang MH, Gu ML, Han C, Guo XJ, Yin GP, Yu P, Kong LY (2018) Aureochaeglobosins A-C, three [4 + 2] adducts of chaetoglobosin and aureonitol dserivatives from Chaetomium globosum. Org Lett 20(11):3345–3348
Zhang YL, Li S, Jiang DH, Kong LC, Zhang PH, Xu JD (2013) Antifungal activities of metabolites produced by a termite-associated Streptomyces canus BYB02. J Agric Food Chem 61:1521–1524
Zhao SS, Zhang YY, Yan Y, Cao LL, Xiao Y, Ye YH (2017) Chaetomium globosum CDW7, a potential biological control strain and its antifungal metabolites. FEMS Microbiol Lett 364(3):1–6
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21602152), Shandong Provincial Natural Science Foundation (ZR2016BB01), Shandong Provincial Key Laboratory of Agricultural Microbiology Open Fund (SDKL2017015).
Author information
Authors and Affiliations
Contributions
PL, DZ and RS performed the experiments and analyzed data. ZY and FZ edited the manuscript. YT designed the experiments. All authors revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, P., Zhang, D., Shi, R. et al. Antimicrobial potential of endophytic fungi from Astragalus chinensis. 3 Biotech 9, 405 (2019). https://doi.org/10.1007/s13205-019-1948-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1948-5