Abstract
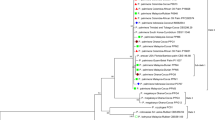
Calendula officinalis plants with phyllody symptoms (CaoP) were observed in Yazd and Ashkezar (Yazd province, Iran) during 2013–2016. Twenty-one symptomatic and four asymptomatic plants were transferred individually to the greenhouse and potted for the biological and molecular characterization of associated phytoplasma. The dodder transmission from symptomatic potted marigold plants, induced virescence, phyllody and witches’ broom symptoms in periwinkle. Total DNAs extracted from symptomatic and symptomless plants and dodder-inoculated periwinkles were subjected to nested PCR assay using primer pairs amplifying phytoplasma ribosomal DNA. Expected PCR amplification was detected in all CaoP plant and dodder-inoculated periwinkle samples. RFLP analysis of the amplicons obtained in direct PCR with P1/P7 primers using RsaI, AluI, MseI, HinfI and HaeIII restriction enzymes showed profiles identical to each other and related to phytoplasmas in all the 21 positive samples. R16mF2/R16mR2 amplicons from six CaoP plant samples were sequenced where consensus sequences had 100% of identity among each other. R16F2n/R16R2-trimmed sequences (1248 bp) of representative samples from Yazd and Ashkezar were deposited in GenBank under accession numbers KU297202 and MH065715, respectively. BLAST search and phylogenetic analysis showed that the CaoP phytoplasma had 99% homology and clusters with phytoplasmas in group 16SrII. Computer-simulated analysis using iPhyClassifier suggests that the CaoP RFLP 16S rRNA gene pattern was identical to 16SrII-D phytoplasmas subgroup. Phytoplasma strains (16SrII-D) were reported as alfalfa witches’ broom disease agent previously in the same geographic areas, and it is possible that alfalfa plays a role in the epidemiology of CaoP disease or vice-versa.




Similar content being viewed by others
References
Asghari Tazehkand S, Hosseini Pour A, Heydarnejad J, Massumi H, Azadvar M (2010) Identification of phytoplasmas associated with cultivated and ornamental plants in Kerman province. Iran J Phytopathol 158(11–12):713–720
Babaie G, Khatabi B, Bayat H, Rastgou M, Hosseini A, Salekdeh GH (2007) Detection and characterization of phytoplasma infecting ornamental and weed plants in Iran. J Phytopathol 155(6):368–372
Bertaccini A, Duduk B (2009) Phytoplasma and phytoplasma diseases: a review of recent research. Phytopathol Mediterr 48:355–378
Bertaccini A, Duduk B, Paltrinieri S, Contaldo N (2014) Phytoplasmas and phytoplasma diseases: a severe threat to agriculture. Am J Plant Sci 5:1763–1788
Chaturvedi Y, Rao G, Tiwari A, Duduk B, Bertaccini A (2010) Phytoplasma on ornamentals: detection, diversity and management. Acta Phytopathol Entomol 45(1):31–69
Deng S, Hiruki C (1991) Amplification of 16S rRNA genes from culturable and non-culturable mollicutes. J Microbiol Methods 14:53–61
Esmailzadeh Hosseini SA, Salehi M, Firooz R, Shamszadeh M (2008) Occurrence of marigold phyllody in Yazd province. In: 18th Iranian plant protection congress. Faculty of Agriculture, University of Bu-Ali Sina, Hamedan, Iran, p 418
Esmailzadeh Hosseini SA, Salehi M, Khanchezar A, Shamszadeh M (2011) The first report of a phytoplasma associated with pot marigold phyllody in Iran. Bull Insectol 64(Suppl):109–110
Esmailzadeh Hosseini SA, Khodakaramian G, Salehi M, Fani SR, Bolok Yazdi HR, Raoufi D, Jadidi O, Bertaccini A (2015a) Status of alfalfa witches’ broom phytoplasma disease in Iran. Phytopathogenic Mollicutes 5(1-Suppl):65–66
Esmailzadeh Hosseini SA, Khodakaramian G, Salehi M, Fani SR, Mirchenari SM, Salehi E, Bertaccini A (2015b) Incidence, distribution and economic importance of alfalfa witches’ broom disease in Sistan–Baluchestan (Iran) and characterization of associated phytoplasma. Phytopathogenic Mollicutes 5(2):84–90
Esmailzadeh Hosseini SA, Salehi M, Khodakaramian G, Mirchenari SM, Bertaccini A (2015c) An up to date status of alfalfa witches’ broom disease in Iran. Phytopathogenic Mollicutes 5(1):9–18
Esmailzadeh Hosseini SA, Khodakaramian G, Salehi M, Bertaccini A (2016a) Characterization of 16SrII group phytoplasmas associated with alfalfa (Medicago sativa) witches’ broom disease in diverse areas of Iran. J Crop Prot 5(4):581–590
Esmailzadeh Hosseini SA, Khodakaramian G, Salehi M, Bertaccini A (2016b) First report of 16SrVI-A and 16SrXII-A phytoplasmas associated with alfalfa witches’ broom diseases in Iran. J Plant Pathol 98(2):369
Esmailzadeh Hosseini SA, Khodakaramian G, Salehi M, Bertaccini A (2016c) Molecular identification and phylogenetic analysis of phytoplasmas associated with alfalfa witches’ broom diseases in the western areas of Iran. Phytopathogenic Mollicutes 6(1):16–22
Esmailzadeh Hosseini SA, Salehi M, Mirchenari SM, Bertaccini A (2016d) First report of a 16SrII-D phytoplasma associated with Calendula officinalis phyllody in Iran. New Dis Rep 34:22
Fattahi M, Salehi M, Sharzehi A, Esmailzadeh Hosseini SA (2016) Partial biological and molecular characteristics of a phytoplasma associated with Behshahr (Mazandaran) periwinkle phyllody. Iran J Plant Pathol 52(1):135–141
Gera A, Maslenin L, Rosner A, Zeidan M, Weintraub PG (2006) Phytoplasma disease in ornamental crops in Israel. Acta Hortic 722:155–162
Gundersen DE, Lee I-M (1996) Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer sets. Phytopathol Mediterr 35:144–151
Khan AJ, Botti S, Al-Subhi AM, Gundersen-Rindal DE, Bertaccini AF (2002) Molecular identification of a new phytoplasma associated with alfalfa witches’ broom in Oman. Phytopathology 92:1038–1047
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Lee I-M, Gundersen-Rindal DE, Davis RE, Bartoszyk IM (1998) Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences. Int J Syst Evol Microbiol 48:1153–1169
Lee I-M, Davis RE, Gundersen-Rindal DE (2000) Phytoplasma: phytopathogenic mollicutes. Ann Rev Microbiol 54:221–255
Marcone C, Ragozzino A, Seemüller E (1997) Detection and identification of phytoplasmas in yellows-diseased weeds in Italy. Plant Pathol 46:530–537
Mirzaie A, Esmailzadeh Hosseini SA, Jafari-Nodooshan A, Rahimian H (2007) Molecular characterization and potential insect vector of a phytoplasma associated with garden beet witches broom in Yazd, Iran. J Phytopathol 155(4):198–203
Omar AF, Alsohim AS (2016) Identification of new plant hosts of 16SrII group phytoplasmas in Saudi Arabia. Phytopathogenic Mollicutes 6(2):71–76
Rani A, Misra P, Singh J, Kumar P, Rani R, Shukla P (2014) PCR based detection of phytoplasma association in pot marigold (Calendula officinalis L.) and guldawari (Dendranthema grandiflora L.). Asian J Biol Sci 9(2):238–241
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Salehi M, Izadpanah K, Siampour M, Esmailzadeh Hosseini SA (2011) Polyclonal antibodies for the detection and identification of Fars alfalfa witches’ broom phytoplasma. Bull Insectol 64(Suppl):59–60
Salehi E, Salehi M, Taghavi SM, Izadpanah K (2014) 16SrII-D phytoplasma strain associated with tomato witches’ broom in Bushehr province. Iran J Crop Prot 3(3):377–388
Salehi M, Esmailzadeh Hosseini SA, Salehi E (2015a) Characterisation of a phytoplasma associated with sunflower phyllody in Fars, Isfahan and Yazd provinces of Iran. New Dis Rep 3:6
Salehi M, Siampour M, Esmailzadeh Hosseini SA, Bertaccini A (2015b) Characterization and vector identification of phytoplasmas associated with cucumber and squash phyllody in Iran. Bull Insectol 68(2):311–319
Salehi M, Esmailzadeh Hosseini SA, Rasoulpour R, Salehi E, Bertaccini A (2016a) Identification of a phytoplasma associated with pomegranate little leaf disease in Iran. Crop Prot 87:50–54
Salehi M, Esmailzadeh Hosseini SA, Salehi E, Bertaccini A (2016b) Molecular and biological characterization of a 16SrII phytoplasma associated with carrot witches’ broom in Iran. J Plant Pathol 98(1):83–90
Salehi M, Esmailzadeh Hosseini SA, Salehi E, Bertaccini A (2016c) Genetic diversity and vector transmission of phytoplasmas associated with sesame phyllody in Iran. Folia Microbiol 62(2):99–109
Salehi M, Esmailzadeh Hosseini SA, Salehi E, Bertaccini A (2016d) Occurrence and characterization of a 16SrII-D subgroup phytoplasma associated with parsley witches’ broom disease in Iran. J Phytopathol 164(11–12):996–1002
Schneider B, Seemüller E, Smart CD, Kirkpatrick BC (1995) Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas. In: S Razin, Tully JG (eds) Molecular and diagnostic procedures in mycoplasmology. Academic Press, San Diego, pp 369–380
Wang K, Hiruki C (2001) Use of heteroduplex mobility assay for identification and differentiation of phytoplasmas in the aster yellows group and the clover proliferation group. Phytopathology 91:546–552
Zhang YP, Uyemoto JK, Kirkpatrick BC (1998) A small-scale procedure for extracting nucleic acids from woody plants infected with various phytoplasmas for PCR assay. J Virol Methods 71:45–50
Zhao Y, Wei W, Lee I-M, Shao J, Suo X, Davis RE (2009) Construction of an interactive online phytoplasma classification tool, iPhyClassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII). Int J Syst Evol Microbiol 59:2582–2593
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Esmailzadeh Hosseini, S.A., Salehi, M., Babaie, G. et al. Characterization of a 16SrII subgroup D phytoplasma strain associated with Calendula officinalis phyllody in Iran. 3 Biotech 8, 295 (2018). https://doi.org/10.1007/s13205-018-1320-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1320-1