Abstract
This study reports an industrially applicable non-sterile xylitol fermentation process to produce xylitol from a low-cost feedstock like corn cob hydrolysate as pentose source without any detoxification. Different immobilization matrices/mediums (alginate, polyvinyl alcohol, agarose gel, polyacrylamide, gelatin, and κ-carrageenan) were studied to immobilize Candida tropicalis NCIM 3123 cells for xylitol production. Amongst this calcium alginate, immobilized cells produced maximum amount of xylitol with titer of 11.1 g/L and yield of 0.34 g/g. Hence, the process for immobilization using calcium alginate beads was optimized using a statistical method with sodium alginate (20, 30 and 40 g/L), calcium chloride (10, 20 and 30 g/L) and number of freezing–thawing cycles (2, 3 and 4) as the parameters. Using optimized conditions (calcium chloride 10 g/L, sodium alginate 20 g/L and 4 number of freezing–thawing cycles) for immobilization, xylitol production increased significantly to 41.0 g/L (4 times the initial production) with corn cob hydrolysate as sole carbon source and urea as minimal nutrient source. Reuse of immobilized biomass showed sustained xylitol production even after five cycles.
Similar content being viewed by others
Introduction
The increasing demand and exorbitant cost of low calorie polyol like xylitol open up challenge for making low-cost xylitol from renewable feedstock. Xylitol, a naturally occurring sugar alcohol sweetener, that has sweetness similar to sucrose but 40 % lower energy, negative heat of dissolution, low viscosity in solution, absence of the Maillard reaction, higher chemical stability, and several biomedical properties (Bär et al. 1991). Emil Fisher was the first to synthesize xylitol by reacting xylose with sodium amalgam in 1891 (Bär et al. 1991). Xylitol is beneficial for nutrition (Sreenivas-Rao et al. 2006), for prevention of dental caries (Emidi 1978) and low-calorie food preparation for diabetic patients (Pepper and Olinger 1988) but these applications are limited due to high cost of xylitol produced by chemical means. The selling cost for xylitol is 6–7 $/kg for bulk purchase (Rafiqul and Sakinath 2013). Xylitol is currently manufactured by chemical hydrogenation of pure d-xylose in the presence of nickel catalyst at elevated temperature and pressure, yielding a product with a high purity (>99.5 %) and a yield of 50–60 % with respect to the initial xylose (Dieters 1975; Ojamo et al. 2009). Alternatively, xylitol can be produced by biological process which shows certain advantages like milder conditions of pressure, temperature, pH, agitation, cell inhibitors and lower costs of downstream processing due to the production of lower amounts of by-products (Saha 2003). However, carbon source and operating cost must be economically competitive to ensure the feasibility of the process. Corn cob, a major waste obtained in corn production and is a promising and attractive alternative for xylose rich hemicellulosic hydrolysate stream (Rivas et al. 2003).
While comparing with the free cell system, the immobilized microorganism system can be used to improve the fermentation performance and to reduce the overall production costs (Roberto et al. 1991). Immobilized cell bioprocesses becomes obvious and most preferred solution because they allow higher fermentation rates, permit high cell concentration, reuse of cells for extended time, reduce costs related to inoculum development, protect the entrapped biocatalyst from inhibitors, prevent washout and provide ease of separation of biocatalyst from fermentation broth (Jirku et al. 2000). Cell immobilization via gel entrapment is widely used in bench-scale tests, and many gel-like materials are used as carriers, which may be based on natural (alginate, κ-carrageenan, agarose, agar, chitosan, etc.) or synthetic (polyacrylamide, polyacrylate, polyurethane, etc.) polymers or precursors (Lozinsky et al. 1997). Among these supports screened for yeast cell immobilization to perform xylose-to-xylitol bioconversion, calcium alginate support has received more attention in biomedical and food industries due to properties viz. integrity, minimal mass transfer limitation, cheap, non-toxic, mild conditions and utilizes ingredients that are accepted as food additive (Champagne et al. 2000). The productivity of immobilized system is affected by three parameters, i.e. calcium chloride, sodium alginate and number of freezing–thawing cycles (Cunha et al. 2009). However, limited number of studies report optimized conditions for immobilization of Candida and even so, most of these studies use detoxified hydrolysate for fermentation (Wang et al. 2009; Carvalho et al. 2003, 2004; Cheng et al. 2009; El-Batal and Khalaf 2004; Liaw et al. 2008; Deng et al. 2006; Sarrouh et al. 2007; Gyan et al. 2011). Inclusion of the detoxification step results in an increase in the overall production cost. It is therefore, desirable to use non-detoxified hydrolysate. Hence, the present work was undertaken with an objective to enhance xylitol production from non-detoxified corn cob hydrolysate using statistically optimized immobilized process for entrapping the cells.
Materials and methods
Raw material and chemicals
Corn cob was provided by Abhyoday feedstock suppliers, Jalgaon, India. These are air-dried and milled into small particles (3.5 mm × 2.5 mm) before acid hydrolysis. Aminex column HPX87H (300 mm × 7.8 mm) was purchased from Biorad, Hercules, CA, USA. All used chemicals were of analytical grade. Sodium alginate, calcium chloride and sodium citrate were procured from Himedia, Mumbai.
Preparation of corn cob hydrolysate
The composition of corn cob used in this study contained 3–5 % w/w of moisture, and the remaining dry matter was made up 60 % w/w of total reducing sugars, of which 25.0 % w/w hemicellulose and 35 % w/w cellulose, 23.0 % w/w lignin and 3 % w/w ash, 3.0 % w/w protein, 1.0 % w/w uronic acid and 2.0 % acetates.
Corn cob contains various components such as lignin, cellulose, hemicellulose, various extractives and inorganic components. Corn cob hydrolysate was obtained by Praj patented pretreatment procedure (Pal et al. 2013). This pretreatment involved corn cob solid of 15–20 % w/w, dilute acid concentration of 1–3 % w/w, screw speed of 2–4 rpm, reaction temperature of 160–180 °C and reaction time of 15–20 min. Then the corn cob slurry and aqueous solution after reaction were discharged, and separated in a solid–liquid separation equipment. The obtained supernatant was rich in xylose concentration, and the main composition was (w/w) xylose 4–6 %, glucose 0.5–1.0 %, arabinose 0.1–0.3 %, acetic acid 0.2–0.6 %, furfural 100–300 ppm, hydroxy methyl furfural 100–500 ppm, galactose 0 %, mannose 0 % and phenolics 2500–4000 ppm.
Microorganism and inoculum cultivation
Candida tropicalis NCIM 3123 was purchased from National Collection of Industrial Microorganism (NCIM), Pune and maintained on MXYP agar slants (containing malt extract, 3 g/L; xylose, 20 g/L, yeast extract, 3 g/L, peptone, 5 g/L and agar, 20 g/Lat pH 7.0) at 4 °C. The microorganism was sub cultured every 2 weeks. A loopful of C. tropicalis strain from the 24-h-old slants maintained on MXYP agar slants were inoculated into 50 mL of inoculum medium containing (g/L) xylose, 20.0; yeast extract, 3.0; malt extract, 3.0; and peptone, 5.0 at pH 6.5 in 250 mL Erlenmeyer flask and was allowed to incubate for 24 h at 30 °C with agitation at 150 rpm. Harvested culture broth was centrifuged at 10,000 rpm for 10 min at 4 °C temperature. The supernatant was removed and the cell pellet was washed twice with sterile distilled water and further used for immobilization studies.
Preparation of immobilized cells
Entrapment in sodium alginate
An adequate volume of cell suspension was added to sterilized 20 mL of 4 % (w/v) sodium alginate prepared in distilled water to get cell concentration of 6 g/L. The slurry was extruded through a syringe into 0.2 M calcium chloride solution with constant stirring. The beads were allowed to cure in 0.2 M calcium chloride solution for 24 h at 4 °C followed by washing with sterile distilled water 3–4 times, and they were preserved in sterile distilled water until use.
Entrapment in polyvinyl alcohol
Sterile aqueous solution containing 2 % w/v sodium alginate and 11 % w/v polyvinyl alcohol were mixed with the thick cell suspension so as to reach a cell concentration of 6 g/L (dry weight). The mixture was then passed through syringe needle (18 gauge) into calcium chloride (40 g/L) with use of peristaltic pump. The beads were maintained in the calcium chloride solution at 4 °C for 3 h. Afterwards, they were washed with sterile distilled water.
Entrapment in agarose gel
An adequate yeast cell suspension was added to the sterile 3 % w/v agarose solution maintained at 40 °C to reach concentration of 6 g/L, mixed well with stirring and poured into sterile petri plate and allowed to solidify. The resultant block was cut into equal size cubes and added to sterile distilled water and kept in refrigerator (1 h) for curing. After curing, sterile water was decanted and the cubes were then washed 3–4 times with sterile distilled water and stored in same for further use.
Whole cell immobilization in polyacrylamide
An adequate yeast cell suspension was mixed with 15 mL of 12 % w/v polyacrylamide solution, 15 mL of distilled water, 3 mL sterile water, 200 μL ammonium persulphate (0.4 gm/mL), and 50 μL TEMED to reach cell concentration of 6 g/L and allowed to polymerize on a sterile petri plate for 1 h. The polymer sheet was cut into cubes (4 mm), rinsed in sterile distilled water and stored at 4 °C for curing for 3 h. The cubes were then washed 3–4 times with sterile distilled water and stored in same till further use.
Whole cell immobilization in gelatin
An adequate volume of yeast cell suspension was added to 15 mL of 6 % w/v sterile gelatin maintained at 45 °C, and poured into a sterile petri dish. The gel was over layered with 10 μL of 5 % w/v glutaraldehyde for hardening at 30 °C. The resulting block was cut into small-size cubes (4 mm) and the cubes were washed thoroughly with sterile distilled water for complete removal of excess glutaraldehyde. The cubes were then washed 3–4 times with sterile distilled water and stored in same till further use.
Whole cell immobilization in κ-carrageenan
Saline solution containing 4 % w/v κ-carrageenan was heated to 60 °C for complete dissolution, and allowed to cool at 40 °C. Then, it was mixed with an adequate volume of yeast cell suspension to get cell concentration of 6 g/L at 32–35 °C without affecting cell viability. The mixture was pumped slowly to 2 % w/v KCl solution to induce gelation. The gel were then washed 3–4 times with sterile distilled water and stored in sterile water till further use.
Screening of different matrices for immobilization of Candida tropicalis cells for xylitol production
The fermentation performance and bead integrity evaluation was performed by direct contact with non-detoxified stream of corn cob pentose hydrolysate. Fermentation was carried out in 500 mL Erlenmeyer flask containing 200 mL of fermentation media. This media consists of xylose concentration of 40–60 g/L for respective stream with 500 ppm urea as nutritional component. Initial pH of media was adjusted to 6.5 using calcium hydroxide. The fermentation was carried out on rotary shaker at 150 rpm and 30 °C. The quantity of immobilised beads was added as inoculum in different flask so that each flask contains equal number of cells like that of free cell fermentation as control.
Optimization of immobilization condition in sodium alginate by statistical method
The yeast cells were immobilized by encapsulation in calcium alginate beads by use of freezing–thawing method (Levia et al. 2013). An adequate volume of cell suspension was added to sterile 20 mL of 20, 30 and 40 g/L of sodium alginate prepared in distilled water to get final cell concentration of 6 g/L. The slurry was extruded through a syringe into calcium chloride solution of 10, 20 and 30 g/L with constant stirring. The beads were allowed to cure in calcium chloride solution for 24 h at 4 °C followed by washing with sterile distilled water 3–4 times, and submitted to the freezing–thawing cycles at −20 °C. The levels for each variable used are shown in Table 1.
A 23 level full factorial design with 8 axial point and 3 replicates at the center point with total of 11 experiments was employed (Box et al. 1978) This design was used to select the optimal immobilization conditions like concentration of sodium alginate, calcium chloride and number of freezing–thawing cycles, to enhance the fermentation performance, bead integrity and to reduce mass transfer limitation. As mentioned in Table 1, selected three independent factors were controlled at two levels (−, +), namely 20 and 40 g/L for the sodium alginate concentration (SA), 10 and 30 g/L for the calcium chloride concentration (CC), and (2 and 4) for the number of freezing–thawing cycles (FTN). These factors and levels were suggested by previous results obtained with Candida spp. cell and yeast cells immobilized onto other supports, polyvinyl alcohol (Cunha et al. 2007, 2009), polyurethane foam (Wang et al. 2009) calcium alginate (Carvalho et al. 2005; Milessi et al. 2013; Chen et al. 2012; Lotfipour et al. 2012) under different conditions.
Fermentation media conditions for statistical design of experiments
The non-detoxified stream was heated at 80 °C for 10 min after pH adjustment to 6.5 with calcium hydroxide and supplemented with 500 ppm urea as a nitrogen source. Batch fermentation were carried out in duplicate in Erlenmeyer flask containing 100 mL fermentation media with corn cob hydrolysate in 250 mL flask and 15 g of wet beads containing cells. The flasks were maintained on rotary shaker at 150 rpm and 30 °C. The effects of the immobilization variables on xylose-to-xylitol conversion as well as on the encapsulation efficiency by the support were investigated through statistical concepts using the Statgraphics program (version 16.0). To this purpose, the yield of xylitol on consumed xylose (Y p/s), the xylitol volumetric productivity (Q P), and the encapsulation efficiency (EE) were selected as the responses. Y p/s was calculated as the ratio of amount of xylitol produced at the end of fermentation to the amount of xylose consumed, Q P as the ratio of maximum xylitol concentration to the fermentation time, The immobilization efficiency (Y i, %) was calculated as the ratio of concentration of immobilized cells (X i, g/L) to the concentration of total (suspended plus immobilized) cells (X T, g/L) and multiplying by 100.
The fermentation efficiency of xylose to xylitol was calculated as the ratio of the net amount of xylitol produced (g/L) to the initial xylose (g/L) of the medium, The conversion efficiency was calculated as the ratio of difference between initial xylose (g/L) and the residual xylose (g/L) to the initial xylose (g/L) in the medium.
Performance evaluation studies in shake flask
The calcium alginate beads were prepared using the optimum concentration of sodium alginate 20 g/L, calcium chloride 10 g/L and 4 number of freezing–thawing cycles at −20 °C. The fermentation performance was evaluated in 500 mL shake flask containing 200 mL of non-detoxified hydrolysate media (55–60 g/L of xylose, 500 ppm urea and pH was adjusted to 6.5 with calcium hydroxide). Media was sterilized at 80 °C for 10 min in a vertical autoclave (Equitronics, Media instruments, Mumbai). Media was inoculated with 15 g of wet beads and operated at agitation of 150 rpm and temperature of 30 °C. Samples were periodically removed and analyzed for xylose and xylitol.
Recyclability study of beads in non-detoxified pentose hydrolysate
The treated hydrolysate was heated at 80 °C for 10 min and supplemented with urea (0.5 g/L), before being used as a fermentation medium. pH was adjusted to 6.5 with calcium hydroxide. Duplicate repeated-batch fermentation runs were carried out in 250-mL Erlenmeyer flasks containing and 100 mL of fermentation medium and 10 g of immobilized biocatalysts. The flasks were maintained in a rotary shaker at 150 rpm and 30 °C for 96 h. After each run/cycle, the fermented medium was discarded, the solid carrier with immobilized cells were carefully drained and gently washed with water to eliminate all non-adhering yeast cells and the immobilized biocatalysts were charged with fresh medium. At the end of each cycle amount of xylitol produced was estimated and the process was carried out using the same immobilized cells for successive cycles.
Analysis
Cell concentration in the resulting suspension was determined by optical density (OD) measurements at 640 nm, using a spectrophotometer, model U-2900 (Hitachi, Tokyo, Japan). The control was the fermentation medium without cells and corn cob hydrolysate particles to avoid any interference of solids on the OD measurements. The immobilized cell concentration was estimated by the same method after dissolution of beads in 0.1 M sodium citrate solution.
A previously constructed calibration curve was used to relate the OD measurements to dry cell concentration in samples of both this suspension and those used for inoculum. Sugar and xylitol concentrations were measured by HPLC, system of Agilent Technologies, 1200 series, model 1200 series (Agilent technologies, CA, USA), equipped with an Aminex HPX-87H (300 × 7.8 mm) column (Bio-Rad, Hercules, CA) and a refractive index G 1362 A detector, an G 1316 B column Oven and G 1311 A pump. Samples were previously filtered through 0.22 µ filter and injected in the column under the following conditions: injection volume of 20 µL, column temperature of 45 °C, 0.05 M H2SO4 as the mobile phase used at a flow rate of 0.6 mL/min.
Results and discussion
In this study, yeast immobilization conditions in calcium alginate were established through the factorial design as mentioned earlier. The research work is distributed in three stages. The first stage involved screening of various matrices to produce xylitol by fermentation in non-detoxified corn corb hydrolysate and to select the matrix with best xylitol fermentation efficiency and stability of matrices in maintaining the shape in corn cob hydrolysate media. Second stage dealt with influence of sodium alginate concentration, calcium chloride concentration and number of freezing–thawing cycles on xylitol yield (Y p/s), volumetric productivity (Q P) and encapsulation efficiency (EE) by the support at shake flask level fermentation study and third stage studied the fermentation using immobilized beads prepared using statistically optimized conditions.
Screening of various matrices used for immobilization for Candida tropicalis in non-detoxified corn cob hydrolysate
Figure 1 represents comparative study of stability and fermentation efficiency of immobilized beads or gel with C. tropicalis cells in non-detoxified corn cob hydrolysate for xylitol production. Polymers like sodium alginate, polyvinyl alcohol, agar, gelatin, polyacrylamide and κ-carrageenan were used as immobilization matrices to form beads or gels. Out of these polymers, beads were formed with sodium alginate, polyvinyl alcohol and gel formation done with agar, gelatin, polyacrylamide and κ-carrageenan. Shake flask fermentation study with these matrices in non-detoxified corn cob hydrolysate resulted into dissolution of gels like gelatin and κ-carrageenan just after addition. Polyvinyl alcohol bead, agarose and polyacrylamide gels were dissolved in non-detoxified media in first cycle. Only calcium alginate beads were not dissolved and maintained their spherical shape even after first cycle of reaction. Higher xylitol fermentation efficiency of 30 %, observed with calcium alginate as compared to all other polymers. Further optimization was required to match the efficiency of free cells used as control. Out of these four polymers, calcium alginate beads were selected for further study and optimization due to their integrity, stability, reusability and high fermentation efficiency among all supports.
Beads preparation and fermentation performance evaluation
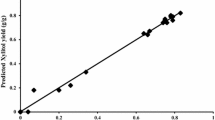
Bead preparation was carried out using full factorial design. The obtained responses indicated that the beads obtained in each run were effective to perform the bioconversion and to maintain the integrity in non-detoxified corn cob hemicellulosic hydrolysate. One of the important point observed was that encapsulation efficiency (EE) observed in each run was more than 99.0 %. The fermentation performance obtained for each run was as shown in Table 1. The xylitol yield, volumetric productivity, and encapsulation efficiency within the pellet were found to be varying in the ranges 0.40–0.74 g/g, 0.17–0.40 g/L/h, and 99.21–99.94 %, respectively.
Statistical data analysis
Figure 2 shows the Pareto charts for each response variable. From analysis of Pareto charts and Anova table it can be concluded that xylitol yield, productivity and immobilization efficiency were not influenced by sodium alginate, calcium chloride concentration and freezing–thawing cycle number (p value > 0.05). The p value revealed that xylitol yield and xylitol productivity was only moderately influenced by the freezing–thawing cycle number (80 % < significance < 95 %). Although a very negligible effect of calcium chloride was observed on yield, productivity and immobilization efficiency, moderately significant interactive effect of calcium chloride and freeze thaw cycle no was observed on immobilization efficiency (80 % < significance < 95 %). Insignificant interactive effects were observed for yield and productivity. The optimum levels for the variables obtained by use of statgraphics software were sodium alginate (2 % w/v), calcium chloride (1.0 % w/v) and 4 number of freezing and thawing cycle.
Fermentations with immobilized cell pellet in shake flask
Figure 3a shows the consumption pattern for different sugars like glucose, xylose, arabinose, acetic acid and xylitol production during fermentations performed in shake flask on a medium based on corn cob hemicellulosic hydrolysate and with pellets prepared using sodium alginate concentration of 20 g/L, calcium chloride concentration of 10 g/L and four freezing–thawing cycles at freezing temperature of −20 °C.
Fermentation profile of xylose to xylitol using optimized immobilized beads. a Consumption of xylose, arabinose, glucose and acetic acid as well as xylitol production. b Profile of fermentation performance with immobilized bead in shake flask (initial xylose, 57.2 g/L; pH 5.92; Temperature 30 °C). c Concentration profile of free cells, cells immobilized in the calcium alginate and pH during fermentation of corn cob hemicellulosic hydrolysate. Error bars represent variation between the duplicate trials
As shown in the Fig. 3a glucose was the preferred substrate as compared to xylose. Glucose was consumed within first 12–24 h only. The presence of glucose is responsible for biomass formation (Yahashi et al. 1996), ethanol formation and known to regulate xylitol formation in pentose fermenting yeasts as it represses the synthesis of xylose reductase (Kastener et al. 2001) the main enzyme responsible for xylose-to-xylitol reduction.
As can be observed in Fig. 3a, b, C. tropicalis cells immobilized in sodium alginate beads were able to convert xylose to xylitol during the fermentation in shake flask, consuming around 98 % xylose and accumulating 41 g/L xylitol after 96 h. The values of xylitol yield (0.73 g/g) and volumetric productivity (0.43 g/L/h) obtained in the present work (Table 2) is better than most of the similar studies based on corn cob hydrolysate (Wang et al. 2009; Cheng et al. 2009; Bibbins et al. 2013). El-Batal and Khalaf (2004), reported higher xylitol titer (48 g/L) than the present work. However, the yield was low and the hydrolysate was detoxified prior to fermentation. Production of xylitol with corn cob hydrolysate is usually less as compared to rice straw hydrolysate (Table 3).
Arabinose consumption was slow as compared to xylose, only 40 % of initial arabinose was consumed in 96 h. Same phenomenon was reported by other authors also (Yahashi et al. 1996) Acetic acid level at the beginning of this fermentation (4.6 g/L) was decreased to 2.4 g/L at the 96 h of fermentation. Overall acetic acid was not consumed as initial concentration was more than 2 g/L. Morita and Silva (2000), reported that at acetic acid concentration (>1.0 g/L), part of acid continues to be directed towards the Krebs cycle and the remainder may be utilized by another energy consuming metabolic pathway (Morita and Silva 2000). Cheng et al. (2009) studied the effect of glucose and acetic acid in the corn cob hydrolysate on xylitol production with C. tropicalis, Biomass growth favored by glucose but acetic acid at high concentration (>2 g/L) was inhibitory. Hence this strain was found to be more efficient to consume acetic acid (Cheng et al. 2009).
Cell growth was considered as the increase of the free cells concentration (X f) in the fermentation medium and of immobilized cells in the gel beads (X i). Figure 3c shows that the final concentration of free cell and the immobilized cell concentration at every periodic hours. Immobilized cell concentration decreased up to 24 h and then increased up to 48 h and remained almost constant until the end of the fermentation. Free cell concentration, which shows around 50–60 % of the total biomass at the end of the fermentation. This result suggests that the pores of the gel matrix were saturated by immobilized cells after this short time. A portion of these cells was released from the beads and proliferated in the medium; reaching a maximum concentration of 95 × 106 cells/mL. Similar results were obtained by other researchers also (Bibbins et al. 2013).
Hence, the studied fermentation occurred due to both free cells and immobilized cells, since a fraction of cells was freely suspended in the medium and another one was entrapped in the beads. The coexistence of mixed cultures consisting and free and immobilized cells is also described by several authors working on different immobilization systems (Bibbins et al. 2013; El-Batal and Khalaf 2004 ). This phenomenon might be due to two factors mainly cell leakage from the immobilized beads due to the shearing forces during agitation and the preferential growth of free cells generated from the surface of bead due to favorable substrate and medium conditions. Liouni et al. (2008), attributed the ability of cells located on the periphery of single cells to multiply and released into suspension as free cells. After 72 h of fermentation, free cells concentration maintained almost constant, probably due to oxygen limitation conditions created either by high biomass level or oxygen depletion in the broth. After this period, xylitol accumulated in the medium and reached its maximum concentration (41 g/L) at the end of the run.
Till now, several research studies have been done with hemicellulosic acid hydrolysate from various lignocellulosic materials for xylitol production. A summary of these results are listed in Table 3. A number of previous studies focus only on detoxification of hydrolysate either by over liming (Wang et al. 2009) or combination with other detoxification method such as activated charcoal (Cheng et al. 2009, Liaw et al. 2008) or ion exchange (Carvalho et al. 2004, Gyan et al. 2011) to improve fermentation performance. Detoxification helps to remove toxic inhibitors like phenolics, furfural and 5-hydroxy methyl furfural (HMF). However, detoxification resulted into 5–10 % loss of sugar which adds to process cost. Only few studies have focused on fermenting hydrolysate without detoxification (Huang et al. 2011; Misra et al. 2013; Ping et al. 2013). In the relevant literature collected, the type of the hydrolysate discussed in the studies of converting xylose into xylitol by microorganisms include corn cob, corn fiber, sugarcane bagasse, hardwood, eucalyptus, rice straw, etc. Among which there is a large difference in relevant xylitol yield. The maximum yield from these studies varies widely from 0.40 to 0.73 g/g and productivity from 0.24 to 1.92 g/L/h.
Bibbins et al. (2013), obtained, fermentation of Debaromyces hansenii immobilized in calcium alginate beads in non-detoxified corn cob hydrolysate, resulted into 12.9 g/L of xylitol, 0.53 g/g yield and 0.23 g/L/h productivity in shake flask with use of synthetic nutrients and Misra et al. (2013), studied scale up in the 10 L reactor with working volume of 5 L of corn cob hemicellulosic hydrolysate without detoxification and adapted C. tropicalis cells to yield product titer of 11.89 g/L of xylitol, yield of 0.58 g/g, volume productivity 0.28 g/L/h and efficiency of 63.73 %. Kwon et al. (2006) used cell recycle fermentation using hollow fiber membrane to get xylitol productivity of 4.14 g/L/h and 0.82 g/g yield with synthetic pure xylose and Kim et al. (2004) produced xylitol from xylose by cell recycle fermentation of C. tropicalis using chemically defined medium to get 5.4 g/L/h productivity and 0.81 g/g yield. Whereas in this study yield, productivity and xylitol titer of 0.73 (theoretically 0.80) g/g, 0.43 g/L/h, 42 g/L were obtained, respectively from corn cob hydrolysate (hydrolysate contained 55–60 g/L xylose, 4.6 g/L acetic acid, 0.30 g/L of furfural and 0.20 g/L of 5-HMF) without any detoxification of hydrolysate or purification of xylose.
Reuse of immobilized cells
Candida tropicalis cells were immobilized in Ca-alginate matrix as described in “Materials and methods”. Immobilized cells were used as inoculums for recycling batches. Such reuse was performed five times without impairment in the bioconversion rates and yields. The immobilized yeast produced xylitol with an average productivity of 0.32 g/L/h and theoretical conversion efficiency of 92 % for five successive batches (Fig. 4). The possibility of producing xylitol with Ca-alginate entrapped cells in synthetic xylose solutions (Misra et al. 2013; Carvalho et al. 2003) and in lignocellulosic hydrolysate (Misra et al. 2013) has been previously demonstrated.
Candia tropicalis NCIM 3123 used in this study showed good potential for xylitol production in producing good xylitol yield and concentration in non-detoxified hydrolysate. More importantly, there was no breakage or disruption of calcium alginate beads in fermentation, so such cells of C. tropicalis immobilized in calcium alginate showed high stability and high performance for xylitol production from corn cob hemicellulosic hydrolysate. The preliminary study presented here demonstrate that the potential of high inhibitor tolerance of C. tropicalis NCIM 3123 when utilized with non-detoxified hydrolysate. In the future optimization of fermentation condition and media optimization for the yeast needs to be investigated.
Conclusions
Industrially feasible process for production of xylitol from non-detoxified hydrolysate with use of entrapped C. tropicalis is demonstrated. C. tropicalis cells were successfully immobilized in calcium alginate using the freezing–thawing method using a 23 full factorial design. More than 99.50 % immobilization efficiency obtained with beads maintaining size, shape in hydrolysate without any visible wearing. The results obtained in terms of the yield of xylitol on consumed xylose, xylitol volumetric productivity, and immobilization efficiency suggested a sodium alginate concentration of 20 g/L, calcium chloride concentration of 10 g/L and 4 numbers of freezing–thawing cycles at freezing temperature of −20 °C as the most optimized conditions for pellet preparation. Reused immobilized biomass showed sustained xylitol production even after 5 cycles. These results demonstrate the feasibility of the proposed immobilization system to be used in future industrial xylose-to-xylitol production from low cost hemicellulosic hydrolysate.
References
Bär A (1991) Xylitol. In: O’Breen Nabors L, Gelardi RC (eds) Alternative sweeteners, 2nd edn. Marcel Deckker, New York, pp 341–379
Bibbins BP, Salgado JM, Torrado A, Uscanga MGA, Domínguez JM (2013) Culture parameters affecting xylitol production by Debaryomyces hansenii immobilized in alginate beads. Process Biochem 48:387–397
Box GEP, Hunder WG, Hunter JS (1978) Statistics for experiments, 1st edn. Wiley, NY
Carvalho W, Silva SS, Santos JC, Converti A (2003) Xylitol production by Ca alginate entrapped cells: comparison of different fermentation systems. Enzyme Microb Technol 32:553–559
Carvalho W, Canilha L, Mussatto SI, Dragone G, Morales MLV, Solenzal AIN (2004) Detoxification of sugarcane bagasse hemicellulosic hydrolysate with ion-exchange resins for xylitol production by calcium alginate-entrapped cells. J Chem Technol Biotechnol 79:863–868
Carvalho W, Santos JC, Canhila L, Silva SS, Pergo P, Converti A (2005) Xylitol production from SCB hydrolysate: metabolic behaviour of Candida guillermondii cells entrapped in a Calcium alginate. Biochem Eng J 25:25–31
Champagne CP, Blahuta N, Brion F, Gagnon C (2000) A vortex-bowl disk atomizer system for the production of alginate beads in a 1500-liter fermentor. Biotechnol Bioeng 68:681–688
Chen XH, Wang XT, Lou WY, Wu YH, Zong MH, Smith TJ, Chen XD (2012) Immobilization of Acetobacter sp. CCTCC M 209061 for efficient asymmetric reduction of ketone and biocatalyst recycling. Microb Cell Factor 11(119):1–13
Cheng KK, Zhang JA, Ling HZ, Ping WX, Huang W, Ge JP, Xu JM (2009) Optimisation of pH and acetic acid concentration for bioconversion of hemicellulose from corn cobs to xylitol by Candida tropicalis. Biochem Eng J 43:203–207
Cunha MAA, Rodrigues RCB, Santos JC, Converti A, Silva SS (2007) Repeated-batch xylitol bioproduction using yeast cells entrapped in polyvinyl alcohol–hydrogel. Curr Microbiol 54(2):91–96
Cunha MA, Converti A, Santos JC, Ferreira STS, Silva SS (2009) PVA-hydrogel entrapped Candida guilliermondii for xylitol production from sugarcane hemicellulose hydrolysate. Appl Biochem Biotechnol 157:527–537
Deng L, Jiang J, Wang Y (2006) Xylitol production by Candida tropicalis from hemicellulosic hydrolysate of ammonia steeped rice straw. Shipin Yu Fajiao Gongye 32(12):1–4
Dieters W (1975) Xylitol production from d-xylose. Swiss patent, 560175 (clco 7C)
El-Batal AI, Khalaf SA (2004) Xylitol production from corn cobs hemicellulosic hydrolysate by Candida tropicalis immobilized cells in hydrogel copolymer carrier. Int J Agric Biol 6:066–1073
Emidi A (1978) Xylitol its properties and food application. Food Technol 32:20–32
Gyan P, Varma AJ, Prabhune A, Shouche Y, Rao M (2011) Microbial production of xylitol from d-xylose and sugarcane bagasse hemicellulose using newly isolated thermotolerant yeast. Bioresour Technol 102(3):3304–3308
Huang CF, Jiang YF, Guo GL, Hwang WS (2011) Development of a yeast strain for xylitol production without hydrolysate detoxification as part of the integration of co-product generation within the lignocellulosic ethanol process. Bioresour Technol 102:3322–3329
Jing L, Lu L, Chunsheng P, Junping Z, Xiaolin L, Yeming S, Pingkai O, Jingjiang L, Shijie L (2009) Poplar woodchip as a biorefinery feedstock–prehydrolysis with formic/acetic acid/water system, xylitol production from hydrolysate and kraft pulping of residual woodchips. J Biomater Bioenergy 3(1):37–45
Jirku V, Masák J, Cejková A (2000) Yeast cell attachment: a tool modulating wall composition and resistance to 5-bromo-6-azauracil. Enzyme Microb Technol 26:808–811
Kastener JR, Eiteman MA, Lee SA (2001) Glucose repression of xylitol production in Candida tropicalis mixed sugar fermentation. Biotechnol Lett 23:1663–1667
Kim TB, Lee YJ, Kim P, Kim CS, Oh DK (2004) Increased xylitol production rate during long-term cell recycle fermentation of Candida tropicalis. Biotechnol Lett 26:623–627
Kwon SG, Park SW, Oh DK (2006) Increase of xylitol productivity by cell-recycle fermentation of Candida tropicalis using submerged membrane bioreactor. J Biosci Bioeng 101:13–18
Levia S, Djordjvic V, Rajic N, Milivojevic M, Bugarski B, Nedovic V (2013) Entrapment of ethyl vanillin in calcium alginate and calcium alginate/poly(vinyl alcohol) beads. Chem Pap 67(2):221–228
Liaw WC, Chen CS, Chang WS, Chen KP (2008) Xylitol production from rice straw hemicellulose hydrolyzate by polyacrylic hydrogel thin films with immobilized Candida subtropicalis WF79. J Biosci Bioeng 105(2):97–105
Liouni M, Drichoutis P, Nerantzis ET (2008) Studies of the mechanical properties and the fermentation behavior of double layer alginate–chitosan beads using Saccharomyces cerevisiae entrapped cells. World J Microbiol Biotechnol 24:281–288
Lotfipour F, Mirzaeei S, Maghsoodi M (2012) Evaluation of the effect of CaCl2 and alginate concentrations and hardening time on the characteristics of Lactobacillus acidophilus loaded alginate beads using response surface analysis. Adv Pharm Bull 2(1):71–78
Lozinsky VI, Zubov AL, Titola EF (1997) Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 2. Entrapped cells resemble porous fillers in their effects on the properties of PVA Acrogel carrier. Enzyme Microb Technol 20:182–190
Milessi TSS, Antunes FAF, Chandel AK, DaSilva SS (2013) Immobilisation of Scheffersomyces stipitis cells with calcium alginate beads: a sustainable method for hemicellulosic ethanol production from sugarcane bagasse hydrolysate. Bioethanol 002:1–8
Misra S, Raghuwanshi S, Saxena RK (2013) Evaluation of corn cob hemicellulosic hydrolysate for xylitol production by adapted strain of Candida tropicalis. Carbohydr Polym 92:1596–1601
Morita TA, Silva S (2000) Inhibition of microbial xylitol production by acetic acid and its relation with fermentative parameters. Appl Biochem Biotechnol 84–86:801–808
Ojamo H, Penttila M, Heikkila H, Uusitalo J, Ilmen M, Sarkki ML, Vehkomaki ML (2009) Method for the production of xylitol. U.S. Patent, 7, 482,144 B2, January 27
Pal S, Joshi S, Padmanabhan S, Rao R, Kumbhar P (2013) Preparation of lignocellulosic hydrolysate (2053/MUM/2013)
Pepper T, Olinger PM (1988) Xylitol in sugar-free confections. Food Technol 42:98–106
Ping Y, Ling HZ, Song G, Ge JP (2013) Xylitol production from non-detoxified corncob hemicellulose acid hydrolysate by Candida tropicalis. Biochem Eng J 75:86–91
Rafiqul ISM, Sakinath AMM (2013) Process for production of xylitol—a review. Food Rev Int 29(2):127–156
Rivas B, Torres B, Dominguez JM, Perego P, Converti A, Parajo JC (2003) Carbon material and bioenergetic balance of xylitol production from corn cobs by Debaryomyces hansenii. Biotechnol Prog 19:706–713
Roberto C, Felipe MGA, Lacis LS, Silva SS, Mancilha IM (1991) Utilisation of sugar cane bagasse hemicellulosic hydrolysate by Candida guillermondii for xylitol production. Bioresour Technol 36:271–275
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Sarrouh BF, Diego TS, Silvio SS (2007) Biotechnological production of xylitol in a 3-phase fluidized bed bioreactor with immobilised yeast cells in Ca-alginate beads. Biotechnol J 2(6):759–763
Silva CJSM, Roberto IC (2001) Improvement of xylitol production by Candida guilliermondii FTI 20037 previously adapted to rice straw hemicellulosic hydrolysate. Lett Appl Microbiol 32:248–252
Sreenivas-Rao R, Pavana-Jyothi C, Prakasham RS, Sarma PN, Venkateswar-Rao L (2006) Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresour Technol 97:1974–1978
Wang L, Qipeng Y, Zheng C, Xiaoguang F (2009) Polyurethane foam immobilization of Candida tropicalis for xylitol production. Microbiology 36(7):943–948
Yahashi Y, Horitsu H, Kawai K, Suzuki T, Takamizawa K (1996) Production of xylitol from d-xylose by Candida tropicalis, the effect of d-glucose feeding. J Ferment Bioeng 81:148–152
Acknowledgments
The authors gratefully acknowledge the analytical team for their support in analyzing the samples and Siddhartha Pal for providing hemicellulosic hydrolysate stream.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest on publication of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yewale, T., Panchwagh, S., Rajagopalan, S. et al. Enhanced xylitol production using immobilized Candida tropicalis with non-detoxified corn cob hemicellulosic hydrolysate. 3 Biotech 6, 75 (2016). https://doi.org/10.1007/s13205-016-0388-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-016-0388-8