Abstract
Salah Al-Din Provence is an active agriculture and population region. One of its primary water sources is groundwater, which suffers from a lack of information regarding water quality and hydrochemistry. In order to study those missing variables, 27 samples from wells of shallow tubes were collected for analyzing the relevant physicochemical indices that help to produce the Schoeller index, Piper diagram, and Gibbs plot. Piper diagram revealed a hydrochemistry behavior of different values along with the groundwater samples. The chemistry of wells was controlled primarily by the evaporation process according to the Gibbs plot. The values of the Schoeller index of the studied samples stated that 59% of them have disequilibrium in Chloro-Alkaline due to their negative obtained values while the rest of them produced positive estimates, which indicates an exchange reaction of cation–anion basic domination. It was concluded that carbonate and silicate weathering, as well as evaporation, controlled the region’ s hydrochemistry. Using Water Quality Index, groundwater was evaluated for use as drinking water. While using Sodium Adsorption Ratio, Sodium percentage, Residual Sodium Carbonate, Magnesium Hazard, and US salinity diagram were all used for estimating the same water’ s suitability for irrigation. All of those indicators, as well as the Gibbs ratio, show that all 27 samples were unsuitable for both studied usage. In addition, those results indicate that evaporation is a major problem for groundwater in this area. Finally, using cluster analysis it was concluded that there are two types of similarities that indicate different levels of pollution in groundwater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, Iraq was considered one of the arid or semi-arid territories. Water scarcity represented a serious challenge facing any activity that depends on water abundance. This was a result of water resources administration policies of surrounding countries; that is, dam construction and rivers diverting in addition to an emerging problem of water quality deterioration (Ismail et al. 2019; Yürekli et al. 2021). Furthermore, the salinity levels of rivers and lakes were remarkably increased to an extent preventing water treatment plants (WTPs) from dealing with such problems. This returned to the fact that the developing countries are still using traditional techniques that were not designed mainly for desalination (Mahmood et al. 2019).
On the other hand, drought is a serious problem in Iraq, particularly in the southern and central regions of the country. The country has been suffering from a severe water crisis for many years, which has been exacerbated by the effects of climate change (Hamed et al. 2023). Drought and groundwater are closely related in Iraq. The country has been suffering from a severe water crisis for many years, and this has led to a depletion of groundwater resources. The drought has had a significant impact on the groundwater levels in the country. The levels of groundwater have decreased significantly, and many areas have been left without water. This has had a devastating impact on the region’ s agriculture, which is heavily dependent on irrigation (Al-Quraishi et al. 2021; Hasan and Abdullah 2023; Jasim and Awchi 2020).
As a result of those problems, more wetlands turned into deserts with a significant decrease in agricultural fields (Zowain and Ismail 2015). However, the scope of solutions focused on the use of groundwater for various use such as irrigation, which deepened the problem as shallow wells were dug randomly and unprofessionally (Ismail et al. 2020). Alongside with the mentioned reasons, many other ones were interfered and deteriorating groundwater quality. This includes climate change, rising evaporation levels, and unprofessional management of water resources in the entire country of Iraq (Al-Ahmadi 2013; Ghalib 2017; Adimalla et al. 2018; Ismail et al. 2018).
This work deals with assessing the groundwater quality in Tikrit County, located exactly in Salah Al-Din province. The importance of this area comes from the fact that most its population depends on the activity of agriculture. For wider regard, the nature of geochemical properties of groundwater quality in Tikrit area and its suitability for irrigation and human direct consumption should be clearer (Nagarajan et al. 2010; Rao et al. 2012; Jasrotia et al. 2018; Kawo and Karuppannan 2018). In order to complete such job, many tests could be followed. Water ions examination, the techniques of advanced statistics; a case in point, cluster analysis to give more information of water quality in the studied region. Moreover, using multivariate statistical model has been proven in many previous researches for its successful handling of such data over many years (Wunderlin et al. 2001; Simeonova et al. 2003; Shrestha and Kazama 2007; Belkhiri et al. 2010; Ismail et al. 2014, 2015, 2020; Abed et al. 2021).
The goal of this study is to explore the groundwater quality and usability in Tikrit area for irrigation and drinking using WQI and geochemical indicators in addition to studying hydrochemical characteristics for a better understanding of groundwater and the complete geochemical process rolling water chemistry indicators in Tikrit.
Materials and methods
Study area
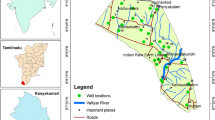
The study area is located in Tikrit, the administrative capital of Salah Al-Din province. This county covers 3539 Km2 of land (Fig. 1). Its geographical location is between a latitude of 36° 0′ 0″ and 34° 16′ 0″ North with a longitude of 43° 0′ 0″ and 44° 0′ 0″ East. It is occupied by 370,000 people (MOP 2007). The general climate of this area tends to be arid to semi-arid. The rainfall averaged to be 14.5 mm (MOT 2010). The air temperature varies from a minimum of 4.6 °C in winter (Dec. to Feb.) to a maximum of 48 °C in summer (Jun. to Aug.) (MOT 2010). The wind is breezing northwesterly most times with an average speed that varies from 1.7 to 3.5 km/h. Humidity; in general, as any of such lands cannot reach saturation. It may reach 68% in its maxima during winter and down to 23% during summer (MOT 2010).
Figure 2 illustrates geological map of the area under consideration. Quaternary (Holocene and Pleistocene) deposit represents the main geological composition in the studied land. It consists mainly of sand dunes, sand sheets, sediments of both valley fill and flood plains, Gypcrete (mainly in a form of gypsiferous as well as Al-Fatha sediments of alluvial fan). Injana formation consists mainly of a sandstone sequence, claystone, and siltstone layers (Jassim and Goff 2006). The aquifer of the studied area consists mainly of Injana formation and Quaternary deposits. The upper secondary layers of groundwater aquifer consists also from Mukdadiya formation which is located between Hamrin hills in the northeast and Tigris River in the west direction. The Outer Platform of Mesopotamia, which is a part of the Arabian Plate, represents the hydrological zones under study (Sissakian and Fouad 2015).
Samples collection and analyzing
Twenty-seven samples were gathered using polypropylene bottles of one liter in volume each. The collection was during March 2020 from shallow tube wells. A continuous pumping was implemented for about ten minutes before taking any sample. Samples locations along the area of study are illustrated in Fig. 1. The parameters of water quality that are taken into consideration were TDS, pH, EC, as well as the following elements K+, Mg2+, Ca2+, Na+ in addition to the radicals of Chloride, Bicarbonate, Carbonate, Nitrate, and Sulfate. The followed analysis procedures were according to some American health institutes (APHA 2005). Some indicators such as EC and pH were tested in situ by portable equipment. The oven of controlled temperature was used to determine TDS. EDTA titration method was used to measure Magnesium (Mg2+) and Calcium (Ca2+). Flame emission photometry was used to estimate Potassium (K+) and Sodium (Na+) concentrations. Chloride ion (Cl−) level was measured by the silver nitrate method. The spectrophotometric method was used to determine Nitrate-Nitrogen (NO3−) and Sulfate (SO42−) levels. Volumetric titration with HCl was used to determine Carbonate (CO32−) and Bicarbonate (HCO3−) concentrations.
Cluster analysis
The method used for evaluating the studied spatial variations along the samples of groundwater sites was cluster analysis which is abbreviated as (CA) and is known a set of statistical multivariate methods. Using such a strategy could present a good explanation for the relationship among the groundwater variables (Ismail and Robescu 2019a). Ward’ s technique of linkage was used to obtain the desired hierarchical clustering. Also, the square Euclidean distance method was followed to determine the distance of similarity and briefed using a dendrogram. In multidimensional space, the geometrical distance is described by the following equation (Singh et al. 2008):
The variables of water quality are required by CA in order to comply with normal distribution. Normality distribution was checked using the test of Shapiro–Wilk. All the observed parameters of water quality are fit to normal distribution excluding TDS, EC, NO3−, SO42−, and K+. As a result, in order to transfer the original figures of non-normal distribution indicators, the form of x′ = log10(x) was used. Upon that, it was discovered that all the studied parameters were normalized excluding NO3−. IBM-SPSS 25 for windows was used to perform the relevant hierarchical CA (Ismail et al. 2018).
Results and discussion
The groundwater hydrochemistry and its general characteristics
Table 1 summarizes the descriptive statistic of the chemical composition of the groundwater in the study area. The values of pH of the area under consideration ranged from 7.11 to 8.18, the mean value estimated to be 7.35, which gives a direct indication of the slight alkalinity of the groundwater.
The registered mean value for TDS as well as EC was found to be 1250 mg/L and 1925 μS/cm respectively. Standard deviation values were 778.24 mg/L and 1120.04 μS/cm with the same respect (Table 1). The high evaporation level may be the reason behind those high values as the study area was described as semi-arid. In the near district of Balad, Ismail et al. (2018) noticed the same high figures. For the cations of K+, Na+, Mg2+, and Ca2+ the mean values were 17.89, 355.44, 100.29, and 197.51 mg/L and the associated standard deviation were 25.90, 135.01, 37.73, and 77.53 mg/L respectively. The spatial variation of TDS and EC in addition to major ions concentrations is illustrated in Fig. 3.
The anions NO3−, HCO3−, SO42−, and Cl− were 3.35, 261.14, 773.81, and 479.40 mg/L. Their standard deviation had the values of 2.4, 126.34, 268.44, and 154.79 mg/L respectively. The carbonate (CO32−) ion concentration held the value of zero in all samples of groundwater. HCO3− can be introduced into the water naturally mainly by CO2 dissolving carbonate-containing minerals or be a result of the combination of CO2 with rainwater (Ismail et al. 2018, 2020).
The indices of Chloro-Alkaline such as CAI1 and CAI2 are used to investigate the ion exchange reaction (Schoeller 1965). The following formulas were used to calculate those indices:
where all values are expressed in milliequivalent per liter. Positive values of CAI1 and CAI2 indicate that Na+ and K+ ions are exchanged with Mg2+ and Ca2+ ions in water. Negative values of these indices indicate that Mg2+ and Ca2+ ions are exchanged with Na+ and K+ from rocks. The values of Schoeller indices for the studies samples in Tikrit showed negative values for 16 samples which indicates that Mg2+ and Ca2+ ions are exchanged with Na+ and K+. The other 11 samples had positive values which changed to Na+ and K+ in water exchanges with Mg2+ and Ca2+ on the rock surface. Calculations for the indices of CAI1 and CAI2 were implemented by Ismail et al. (2018) in Balad, Southern Salah Al-Din province (Fig. 1). It was concluded that 46% of the samples were with negative values of Schoeller indices. Balad area showed a relatively high levels of Na+
The values of hydrochemical evolution of the groundwater were determined using the Piper diagram (Piper 1944) which was implemented using the software of AquaChem (Fig. 4). This software gave the indication that most of the studied groundwater samples fall under three classifications: Ca–Cl, Na–Cl, and Mixed Ca–Mg–Cl type. Those types suggest that surface water is the source of high-salinity mixing water, and the reason behind the remarkable effects of the dispersed evaporates of the aquifer matrix (Ismail et al. 2020). Also, the outcome of Piper diagram complies with the obtained results by Ismail et al. (2019).
Gibbs ratio was used to investigate the controlling mechanism of groundwater chemistry (Gibbs 1970), which could be applied to investigate the most effective sources that indicate the constitutive chemical dissolution (Fig. 5). From this plot, it can be concluded that all the samples of groundwater under consideration were categorized under the zone of evaporation dominance. Also, it is observed that the dominant driving force that controls water chemistry is the evaporation process.
In the current study, a bivariate plot was used to investigate the hydrogeochemical processes. The assessment of carbonate and silicate weathering share was implemented by drawing Total Cations Concentration (TCC) against (Na+ + K+) and (Ca2+ + Mg2+) as illustrated in Fig. 6a, b (Kumar et al. 2008; Das et al. 2016, 2017). The obtained observation was that around 50% of the studied samples were below the 1:2 line. This was a direct indication of the abundance of Ca2+ and Mg2+ as a result of carbonate minerals and reverse ion exchange. The other samples lay along the mentioned line, which shows silicate-weathering dominance. The same behavior was observed by other researchers in Jorhat district in Assam, India as well as Tarmiah district in Iraq (Das et al. 2015; Ismail et al. 2020).
the groundwater hydrochemistry represented in Scatter plots and the spatial trends of carbonate weathering in Tikrit area. a TCC versus ions of Ca2+ and Mg2+, b TCC versus ions of Na+ + K+, c Na+:Cl− ratio in moles versus EC, d Na+ versus Cl−, e NO3− versus K+, f NO3 versus Cl−, g Ca2+/Mg2+ versus Sample No., h (Ca2+ + Mg2+) versus (SO42− + HCO3−), i HCO3− versus Ca2+, j SO42− versus Ca
EC was plotted versus Na+/Cl− molar ratio as shown in Fig. 6c. If this ratio runs near unity, this indicates halite dissolution (Na+ and Cl− will be equally released due to the process). If the above ratio exceeds unity; i.e. higher Na+, an indication that the dominance of silicate weather is obtained. On the other hand, a ratio under unity (higher Cl−) indicates the dominance of the evaporation step associated with the ion exchange of Ca+ reversibly with Na+. Also, the evaporation process could be dominant to an extent that controlling the chemistry of water in case the ratio of Na+/Cl− still unchangeable with increasing EC (Kumar et al. 2006). Figure 6c shows that about 50% of the studied groundwater samples had mostly fixed Na+/Cl− proportion with an increase of EC. In this work, it was concluded that both silicate weathering and evaporation have equal dominance over the study area (Fig. 6d).
As it is shown in Fig. 6e, no relation between NO3− and K+ was observed. The concentrations of NO3− registered very low levels compared to K+. This gave the conclusion that the source of K+ may be the silicate weather, while anthropogenic activities were the source of NO3− (Das et al. 2017). Figure 6f showed a positive relationship between NO3− and Cl−; the indication of this may be that both ions have the same source; i.e. the anthropogenic. Ismail et al. (2020) got the same behavior.
In Fig. 6g the molar ratio of Ca2+/Mg2+ in the samples of groundwater was shown. The first impression indicates the presence of dolomite dissolution and calcite (Kumar et al. 2006). A ratio near unity means dolomite dissolution, while a ratio of Ca2+/Mg2+ ranging from 1 to 2 indicates higher levels of calcite dissolution. A ratio above 2 gave the conclusion that dissolution and silicate weathering is the major process. It was observed that nearly 63% of samples ranged between 1 and 2 in Ca2+/Mg2+ ratio. This indicates that silicate weathering is less dominant than calcite dissolution within the studied area (Das et al. 2016). Figure 6h represents SO42− + HCO3− versus Ca2+ + Mg2+ plot. Around 60% of the studied samples were below the 1:1 line. Because of that, it can be concluded that reverse ion exchange had a slight increase more than the common values.
When plotting Ca2+ versus HCO3−, it appears clear that the majority of samples were under the equal line (Fig. 6i). From this, it is clear that carbonate mineral “calcite” weathering interferes with the release of Ca+2 into the water instead of carbonic acid “silicate” weathering. In addition, when plotting Ca2+ versus SO42−, we obtain that there is an excess amount of SO42− ion over the ion of Ca2+ (Fig. 6j). Moreover, the plot shows that the possible source of Ca2+ ion came from is anhydrite or may be gypsum dissolution, rather than the calcite weathering (Mgbenu and Egbueri 2019).
Groundwater usability
The usability of groundwater was explored using the Water Quality Index (WQI) technique, which is a well-known method that is frequently dealt with for assessing the suitability of groundwater (Vasanthavigar et al. 2010; Rajankar et al. 2011; Nagaraju et al. 2016). WQI is working with a scale between 0 and 100, higher number means better quality and vice versa (Massoud 2012). In addition to drinking water, it can evaluate water quality for various uses such as irrigation and industry (Abed et al. 2021). In works of literature, it could be found many WQI models, which can be frequently in review articles (Lumb et al. 2011; Sutadian et al. 2016). In the current study, the selected index was Bascaron Water Quality Index (BWQI) (Bascaron 1979). The choice was because of its wide usage globally (Pesce and Wunderlin 2000; Debels et al. 2005; Kannel et al. 2007; Massoud 2012; Ismail and Robescu 2019b). For multiple samples, this index can be identified mathematically as:
where n = The sum of variables. Ci = value of the factor i after the appropriate normalization. Pi = proportional weight associated with each factor which has the range of 1–4 depending to its impact on water usability.
In addition to reason above, BWQI was chosen because of its ability to include many variables of water usability for implementing the necessary calculation of the general index after specifying the normalization factors in addition to their weights. Some parameters of water quality are summarized in Table 2 which are normalized and weighted in previous research (Pesce and Wunderlin 2000; Kannel et al. 2007; Massoud 2012). 8 water quality factors were evaluated in this work; that is, NO3, SO4, Cl, Mg, Ca, TDS, EC, and pH. HCO3, Na, and K were excluded in the above calculations because they have no normalized and weighted parameters. This WQI was widely applied in the literature and has the advantage to include many water quality parameters for implementing the necessary calculation of the general index after specifying the normalization factors in addition to their weights. The normalization parameters as well as their relative participation listed in Table 2 were utilized for the chosen factors to get the final BWQI.
The following categories were adopted in order to classify water quality:
-
Excellent: 90–100
-
Good: 71–90
-
Medium: 51–70
-
Bad: 26–50
-
Very bad: 0–25
Table 3 described the calculated sub-indices as well as the final values and categories of the studied water usability, which is found to be medium or bad. Figure 7 illustrates the spatial variation of the studied BWQI.
Quality of groundwater for irrigation
Multiple geochemical factors were evaluated in order to estimate the usability of the groundwater in irrigation. That is, Na percentage, Sodium Adsorption Ratio (SAR), US Salinity Diagram, and Mg Hazards (MH). In addition, EC evaluated the usability of the groundwater under consideration for use in irrigation. MH, %Na, and SAR can be estimated in the following equations:
where all values are expressed in milliequivalent per liter.
The spatial distribution of groundwater usability for irrigation along the area under consideration was shown in Fig. 8. It is obvious that EC has a remarkable impact on defining the level of usability of groundwater in irrigation. According to the obtained results of this parameter, most samples were not suitable for the use of irrigation. On the contrary, MH, %Na, and SAR were not of that unsuitability level. That is, for MH, which is proposed by Szaboles and Darab (1964), Fig. 8 described the majority of samples as suitable for the use of irrigation. %Na results gave the indication that the studied samples were categorized from permissible to good. The US salinity diagram, which is suggested by USSL (1954), and SAR were drawn against EC using AquaChem software as shown in Fig. 9. It was seen that, except for a single sample, all of them lay in C4S1, C4S2, and C4S3 zones which indicates the poor usability of such water for irrigation. The mentioned single excepted sample fell in C3S1 zone and is considered fair for irrigation. The previous works claimed that groundwater is unusable for irrigation in both southern and northern Baghdad, south of Salah Al-Din, and north-eastern Iran (Ghalib 2017; Ismail et al. 2018, 2020).
Multivariate analysis
Hierarchical CA was utilized to estimate the spatial variations along the sites under study and shown as a dendrogram (Fig. 10). Resulted samples were categorized into Group A and Group B. Group A; in turn, was classified into two groups (A1 and A2). Group A1 included 59% of the samples that were linked to comparatively low pollution sites. Group A2 included 34% of samples and was linked to comparatively moderate pollution sites. Group B represented 7% of the samples and was linked to sites with relatively high pollution. Samples were spaced in each group at its place (Fig. 10). This gives the conclusion that the anthropogenic behaviour in the study area may directly affect groundwater quality.
Conclusions
Generally, this work revealed that the area under consideration facing a serious pollution threat. Hydrochemistry of the studied groundwater samples obtained from Piper plot gave 3 types of water which led to conclude that water of high salinity mixture was produced as a result of evaporation and surface water. In Gibbs ratio, most groundwater samples are found in the evaporation dominance zone. This gave the conclusion that groundwater chemistry is extremely affected by evaporation. A percentage of 59% of the studied samples recorded negative values of both CAI1 and CAI2 in ion-exchange reactions. The conclusion that is Mg2+ and Ca2+ exchange are reciprocated with K+ and Na+ ions from rock. The other samples percentage; i.e. 41%, recorded positive values which indicates a direct exchange reaction of base (cation–anion). Anthropogenic activities, carbonate weathering, silicate, and evaporation affect the groundwater hydrogeochemistry. The silicate weathering and evaporation registered the highest influence on the quality of groundwater. Regarding BWQI, the water quality categorization in all samples recorded medium to bad for drinkability. According to the diagram of US salinity, the conclusion was the unsuitability of the studied samples for irrigation. For pollution levels, 3 different of them were observed in hierarchical CA; that is, low, moderate, and high polluted sites. At every site, spaced and studied samples were included in each clustered group.
Data availability
Data are available upon request.
References
Abed BS, Farhan AA-R, Ismail AH, Al Aani S (2021) Water quality index toward a reliable assessment for water supply uses: a novel approach. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03338-7
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process 5(2):363–383. https://doi.org/10.1007/s40710-018-0297-4
Al-Ahmadi ME (2013) Hydrochemical characterization of groundwater in wadi Sayyah Western Saudi Arabia. Appl Water Sci 3:721–732. https://doi.org/10.1007/s13201-013-0118-x
Al-Quraishi AMF, Gaznayee HA, Crespi M (2021) Drought trend analysis in a semi-arid area of Iraq based on normalized difference vegetation index, normalized difference water index and standardized precipitation index. J Arid Land 13:413–430. https://doi.org/10.1007/s40333-021-0062-9
APHA (2005) Standard methods for the examination of water and waste water, 21st edn. American Public Health Association, Washington
Bascaron M (1979) Establishment of a methodology for the determination of water quality. Bol Inf Medio Ambient 9:30–51
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater—a case study: Ain Azel plain (Algeria). Geoderma 159(3–4):390–398. https://doi.org/10.1016/j.geoderma.2010.08.016
Das N, Patel AK, Deka G, Das A, Sarma KP, Kumar M (2015) Geochemical controls and future perspective of arsenic mobilization for sustainable groundwater management: a study from Northeast India. Groundw Sustain Dev 1:92–104
Das N, Deka JP, Shim J, Patel AK, Kumar A, Sarma KP, Kumar M (2016) Effect of river proximity on the arsenic and fluoride distribution in the aquifers of the Brahmaputra floodplains, Assam, Northeast India. Groundw Sustain Dev 2–3:130–142. https://doi.org/10.1016/j.gsd.2016.07.001
Das N, Sarma KP, Patel AK, Deka JP, Das A, Kumar A, Shea PJ, Kumar M (2017) Seasonal disparity in the co-occurrence of arsenic and fluoride in the aquifers of the Brahmaputra flood plains Northeast India. Environ Earth Sci 76:183. https://doi.org/10.1007/s12665-017-6488-x
Debels P, Figueroa R, Urrutia R, Barra R, Niell X (2005) Evaluation of water quality in the Chillan river (Central Chile), using physical and chemical parameters and a modified water quality index. Environ Monit Assess 110:301–322. https://doi.org/10.1007/s10661-005-8064-1
Ghalib HB (2017) Groundwater chemistry evaluation for drinking and irrigation utilities in east Wasit Province Central Iraq. Appl Water Sci 7:3447–3467. https://doi.org/10.1007/s13201-017-0575-8
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090. https://doi.org/10.1126/science.170.3962.1088
Hamed MM, Sammen SS, Nashwan MS et al (2023) Spatiotemporal variation of drought in Iraq for shared socioeconomic pathways. Stoch Environ Res Risk Assess 37:1321–1331. https://doi.org/10.1007/s00477-022-02343-7
Hasan IF, Abdullah R (2023) Multivariate index for monitoring drought (case study, Northeastern of Iraq). Nat Hazards 116:3817–3837. https://doi.org/10.1007/s11069-023-05837-x
Ismail AH, Robescu D (2019a) Application of multivariate statistical techniques in water quality assessment of Danube river. Rom Environ Eng Manag J 18(3):719–726
Ismail AH, Robescu D (2019b) Assessment of water quality of the Danube river using water quality indices technique. Environ Eng Manag J 18(8):1727–1737
Ismail AH, Abed BS, Abdul-Qader S (2014) Application of multivariate statistical techniques in the surface water quality assessment of Tigris River at Baghdad stretch. J Babylon Univ (JUB) 22:450–462
Ismail AH, Muntasir AH, Channo RJ (2015) Groundwater quality assessment in urban area of Baghdad, Iraq, using multivariate statistical techniques. Eng Technol J 33:463–476
Ismail AH, Muntasir AS, Mahmood M (2018) Hydrochemical characterization of groundwater in Balad district Salah Al-Din Governorate Iraq. J Groundw Sci Eng 6:306–322. https://doi.org/10.19637/j.cnki.2305-7068.2018.04.006
Ismail AH, Shareef MA, Alatar FM (2019) Hydrochemistry of groundwater and its suitability for drinking and irrigation in Baghdad Iraq. Environ Process 6:543–560. https://doi.org/10.1007/s40710-019-00374-x
Ismail AH, Hassan G, Sarhan AH (2020) Hydrochemistry of shallow groundwater and its assessment for drinking and irrigation purposes in Tarmiah district, Baghdad governorate, Iraq. Groundw Sustain Dev 10:100300. https://doi.org/10.1016/j.gsd.2019.100300
Jasim AI, Awchi TA (2020) Regional meteorological drought assessment in Iraq. Arab J Geosci 13:284. https://doi.org/10.1007/s12517-020-5234-y
Jasrotia AS, Taloor AK, Andotra U, Bhagat BD (2018) Geoinformatics based groundwater quality assessment for domestic and irrigation uses of the Western Doon valley, Uttarakhand India. Groundw Sustain Dev 6:200–212. https://doi.org/10.1016/j.gsd.2018.01.003
Jassim SZ, Goff JC (2006) Geology of Iraq. Dolin Prague and Moravian Museum, Brno, p 341
Kannel P, Lee S, Lee Y, Kanel S, Khan SP (2007) Application of water quality indices and dissolved oxygen as indicators for river water classification and urban impact assessment. Environ Monit Assess 132:93–110. https://doi.org/10.1007/s10661-006-9505-1
Kawo NS, Karuppannan S (2018) Groundwater quality assessment using water quality index and GIS technique in Modjo river basin, central Ethiopia. J Afr Earth Sci 147:300–311. https://doi.org/10.1016/j.jafrearsci.2018.06.034
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50:1025–1039. https://doi.org/10.1007/s00254-006-0275-4
Kumar M, Kumari K, Singh UK, Ramanathan AL (2008) Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environ Geol 57:873–884. https://doi.org/10.1007/s00254-008-1367-0
Lumb A, Sharma TC, Bibeault JF (2011) A review of genesis and evolution of water quality index (WQI) and some future directions. Water Qual Expo Health 3(1):11–24. https://doi.org/10.1007/s12403-011-0040-0
Mahmood W, Ismail AH, Shareef MA (2019) Assessment of potable water quality in Balad city, Iraq. In: IOP conference series: materials science and engineering, vol 518, p 022002. https://doi.org/10.1088/1757-899X/518/2/022002
Massoud MA (2012) Assessment of water quality along a recreational section of the Damour river in Lebanon using the water quality index. Environ Monit Assess 184:4151–4160. https://doi.org/10.1007/s10661-011-2251-z
Mgbenu CN, Egbueri JC (2019) The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Appl Water Sci 9:22. https://doi.org/10.1007/s13201-019-0900-5
MOP (2007) Ministry of Planning/Salal Al-Din Planning office (unpublished materials)
MOT (2010) Ministry of Transportation/Iraqi Metrological Organization and Seismology (unpublished materials)
Nagarajan R, Rajmohan N, Mahendran U, Senthamilkumar S (2010) Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu India. Environ Monit Assess 171:289–308. https://doi.org/10.1007/s10661-009-1279-9
Nagaraju A, Thejaswi A, Sun L (2016) Statistical analysis of high fluoride groundwater hydrochemistry in Southern India: quality assessment and implications for source of fluoride. Environ Eng Sci 33(7):1–7. https://doi.org/10.1089/ees.2015.0511
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the impact of Cordoba city (Argentina) on Suquia river. Water Res 34(11):2915–2926. https://doi.org/10.1016/S0043-1354(00)00036-1
Piper AM (1944) A graphical procedure in the geochemical interpretation of water. Eos Trans Am Geophys Union 25:914–928. https://doi.org/10.1029/TR025i006p00914
Rajankar PN, Tambekar DH, Wate SR (2011) Groundwater quality and water quality index at Bhandara district. Environ Monit Assess 179:619–625. https://doi.org/10.1007/s10661-010-1767-y
Rao NS, Rao PS, Reddy GV, Nagamani M, Vidyasagar G, Satyanarayana NLVV (2012) Chemical characteristics of groundwater and assessment of groundwater quality in Varaha river basin, Visakhapatnam district, Andhra Pradesh India. Environ Monit Assess 184:5189–5214. https://doi.org/10.1007/s10661-011-2333-y
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and developments. UNESCO. pp 54–83
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin Japan. Environ Model Softw 22:464–475. https://doi.org/10.1016/j.envsoft.2006.02.001
Simeonova P, Simeonov V, Andreev G (2003) Water quality study of the Struma river basin, Bulgaria (1989–1998). Cent Eur J Chem 1:136–212. https://doi.org/10.2478/BF02479264
Singh UK, Kumar M, Chauhan R, Jha PK, Ramanathan AL, Subramanian V (2008) Assessment of the impact of landfill on groundwater quality: a case study of the Pirana site in western India. Environ Monit Assess 141:309–321. https://doi.org/10.1007/s10661-007-9897-6
Sissakian VK, Fouad SFA (2015) Geological map of Iraq, scale 1:1,000,000, 4th edition, 2012. Iraqi Bull Geol Min (IBGM) 11(1):9–16
Sutadian AD, Muttil N, Yilmaz AG, Perera BJC (2016) Development of river water quality indices—a review. Environ Monit Assess 188:58
Szaboles I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th international congress of ISSS, Trans, II. pp 803–812
USSL, US Salinity Laboratory Staff (1954) Diagnosis and improvements of saline and alkali soils. US Department of Agriculture Handbook. No. 60, USDA. p 160
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi RR, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609. https://doi.org/10.1007/s10661-009-1302-1
Wunderlin DA, Diaz MP, Ame MV, Pesce SF, Hued AC, Bistoni MA (2001) Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquia river basin (Cordoba, Argentina). Water Res 35:2881–2894. https://doi.org/10.1016/s0043-1354(00)00592-3
Yürekli K, Erdoğan M, Ismail AH, Shareef MA (2021) How far can hydrochemical characteristics of surface water meet drinking and irrigation criteria: a pragmatic study for the Euphrates river basin, Turkey. Sustain Water Resour Manag 7:25. https://doi.org/10.1007/s40899-021-00507-x
Zowain A, Ismail AH (2015) Management of salinity issues in Iraq’ s agricultural sector using SWOT analysis. Eng Technol J 33:644–658
Acknowledgements
It is our honor and pleasure to thank the General Commission for Groundwater for their valuable help and support.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ismail, A.H., Shareef, M.A., Hassan, G. et al. Hydrochemistry and water quality of shallow groundwater in the Tikrit area of Salah Al Din Province, Iraq. Appl Water Sci 13, 197 (2023). https://doi.org/10.1007/s13201-023-02008-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-02008-y