Abstract
The present study was carried out to find the groundwater quality of coastal aquifer along Manavalakurichi coast. For this study, a total of 30 groundwater samples were collected randomly from open wells and borewells. The concentration of major ions and other geochemical parameters in the groundwater were analyzed in the laboratory by adopting standard procedures suggested by the American Public Health Association. The order of the dominant cations in the study area was found to be Na+ > Ca2+ > Mg2+ > K+, whereas the sequence of dominant anions was \({\text{Cl}}^{ - } > {\text{HCO}}_{3}^{ - } > {\text{SO}}_{4}^{2 - }\). The hydrogeochemical facies of the groundwater samples were studied by constructing piper trilinear diagram which revealed the evidence of saltwater intrusion into the study area. The obtained geochemical parameters were compared with the standard permissible limits suggested by the World Health Organization and Indian Standard Institution to determine the drinking water quality in the study area. The analysis suggests that the groundwater from the wells W25 and W26 is unsuitable for drinking. The suitability of groundwater for irrigation was studied by calculating percent sodium, sodium absorption ratio and residual sodium carbonate values. The Wilcox and USSL plots were also prepared. It was found that the groundwater from the stations W1, W25 and W26 is unfit for irrigation. The Gibbs plots were also sketched to study the mechanisms controlling the geochemical composition of groundwater in the study area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater is an essential source of drinking for numerous people around the world. Saline water intrusion in the coastal aquifers due to overexploitation of groundwater and other anthropogenic activities is a serious environmental issue nowadays. To protect the aquifers from quality degradation, geochemical assessment and monitoring is necessary. It is estimated that approximately one-third of the world’s population use groundwater for drinking purpose (Nickson et al. 2005). In India, more than 90 % of rural and nearly 30 % of urban population depends on groundwater for their drinking and domestic requirements (Jaiswal et al. 2003). According to Babiker et al. (2007), the chemistry of groundwater is not only related to the lithology of the area and the residence time the water is in contact with rock material, but also reflects inputs from the atmosphere, soil and weathering as well as pollutant sources such as saline intrusion, mining, and industrial and domestic wastes. Excessive irrigation activities also resulted in groundwater pollution in India (Pawar and Shaikh 1995; Sujatha and Reddy 2003).
The overexploitation of groundwater along the coastal area has become a major environmental issue in the entire world. In many coastal areas, human settlements together with the day-by-day development of industrial, agricultural and tourist activities have led to overexploitation of aquifers. Because of this exploitation, the chances of intrusion of seawater into the inland aquifer are very high and also affects the quality of groundwater. In the present study, the geochemical characteristics of groundwater is determined to know the groundwater quality along the coastal aquifer of Manavalakurichi, located in Kanyakumari District of Tamil Nadu, India.
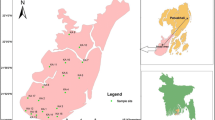
The study area Manavalakurichi is a town panchayat located in the southern end of Tamil Nadu, India. The study area extends from 8°7′ to 8°10′N longitudes and 77°19′ to 77°19′E latitudes. It is connected by road network to Trivandrum, Tirunelveli and Kanyakumari. The study area is situated in Kanyakumari District and the southern side of the study area is bounded by the Indian Ocean and in the west by the Arabian Sea. Manavalakurichi and its surroundings are well known for their heavy mineral deposits. Several mining areas under the control of Indian Rare Earth Limited (IREL) are situated here. The average temperature in the study area varies from 22.8 to 33.6 °C with an average rainfall of about 846–1456 mm. The present study area is shown in Fig. 1. A major part of the study area is geologically covered by Quaternary fluvio-marine deposits. The north and northwestern regions of the district are completely occupied by the Western Ghats Mountain with a maximum elevation of 1658 m (CGWB 2008). The coastal tract in the south of the study area is a thin strip of plain region that has a width of 1–2.5 km (Srinivas et al. 2013) and is mostly covered by marshy swamps and a number of sand dunes (Teri sands). The soil type of the present area is classified into red soil, red lateritic soil, brown soil and coastal sand. The soils of this district are in situ, in nature, pale reddish, lateritic and earthy in color (CGWB 2008).
Methodology
The study area base map was scanned and digitized from the Survey of India (SOI) toposheet No. 58 H/8 (1:50,000) (GSI 1995). ArcGis 9.3 software was used for digitization and to analyze the data for groundwater quality evaluation. Groundwater samples were randomly collected from 30 open wells and borewells during May 2013, representing the premonsoon season (Fig. 1). For sample collection, high-density polyethylene bottles were used. The bottles are immediately sealed after collecting the sample to avoid reaction with the atmosphere. The sample bottles were labeled systematically. The collected samples were analyzed in the laboratory for various physicochemical parameters. During sample collection, handling, preservation and analysis, standard procedures recommended by the American Public Health Association (APHA 1995) were followed to ensure data quality and consistency.
The pH and electrical conductivity (EC) were measured in situ by using Hanna (HI9828 USA) multi-parameter probe, and the major ions (Ca2+, Mg2+, Na+, K+, \({\text{HCO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\), Cl−) were analyzed in the laboratory using the standard methods suggested by the American Public Health Association (APHA 1995). Among the analyzed ions, sodium (Na+) and potassium (K+) were determined using flame photometer. Calcium (Ca2+), magnesium (Mg2+), bicarbonate \(( {\text{HCO}}_{3}^{ - } )\) and chloride (Cl−) were analyzed by volumetric methods and sulfate \(( {\text{SO}}_{4}^{2 - } )\) was estimated using spectrophotometer. The piper diagram, Gibbs plot, Wilcox diagram and the USSL plot were used for studying the quality of the groundwater in detail.
The spatial analysis of various physico-chemical parameters was carried out using ArcGIS 9.3 software. An inverse distance weighed (IDW) algorithm was used to interpolate the data spatially and to estimate the values between measurements. The IDW technique calculates a value for each grid node by examining surrounding data points that lie within a user-defined search radius (Burroughs and McDonnell 1998). All of the data points are used in the interpolation process and the node value is calculated by averaging the weighted sum of all the points.
Results and discussions
The analytical results have been evaluated to find out the suitability of groundwater in the study area for drinking and agricultural uses. By comparing the obtained values of different water quality parameters with the guidelines of the World Health Organization (WHO 2011) and Indian Standard Institute (ISI 1983), we obtain the suitability of groundwater for drinking and agricultural purposes (Table 1).
The concentration and behavior of major ions such as Ca2+, Mg2+, Na+, K+, \({\text{HCO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\)and Cl− and some important physico-chemical parameters such as pH, EC, total dissolved solids (TDS) and the suitability of groundwater in the study area are discussed below.
pH
The balance between the concentration of hydrogen ion and hydroxyl ions in the water is termed as pH. The limit of pH value for drinking water is specified as 6.5–8.5 (WHO 2011; ISI 1983). The pH of water provides vital information in many types of geochemical equilibrium or solubility calculations (Hem 1985). The pH value of most of the groundwater samples in the study area varies from 5.3 to 7.4, which clearly shows that the groundwater in the study area is slightly acidic in nature. This may be attributed to anthropogenic activities such as sewage disposal and use of fertilizers in agricultural lands (paddy fields and coconut plantation) of the study area. It may also be due to natural phenomena like intrusion of brackish water into the sandy aquifer, which initiates the weathering process of the underlying geology (Sarat Prasanth et al. 2012).
Electrical conductivity (EC)
EC is a measure of capacity of water to convey electric current. The most desirable limit of EC in drinking water is prescribed as 1500 µS/cm (WHO 2011). The EC of the groundwater in the study area varies from 67.2 and 2771.8 µS/cm with an average value of 818 µS/cm. Higher EC in few groundwater samples indicates the enrichment of salts in the groundwater. The value of EC may be an approximate index of the total content of dissolved substance in water. It depends upon temperature, concentration and types of ions present (Hem 1985). The effect of saline intrusion may be the reason for medium enrichment of EC in the study area. The effect of pH may also increase the dissolution process, which eventually increases the EC value (Sarat Prasanth et al. 2012).
Calcium and magnesium
The concentration of calcium ions (Ca2+) in the groundwater samples ranges from 6.4 to 124.2 mg/l with an average value of 34.3 mg/l and that of magnesium ions (Mg2+) ranges from 0.5 to 118.2 mg/l (average value of 39.1 mg/l) (Table 1). The principal sources of calcium and magnesium in most of the groundwater samples are detrital minerals such as plagioclase feldspar, pyroxene, amphibole and garnet (Hounslow 1995). The limestone, dolomite, gypsum, anhydrates and clay minerals among the sedimentary rocks of the coastal region also enhance the calcium and magnesium content in the groundwater (Chandrasekar et al. 2013). Reverse cationic exchange, i.e., the replacement of calcium and magnesium ions by sodium ion in the groundwater may be the reason for lower concentration of Ca2+ and Mg2+ in some areas (Jacob et al. 1999). Figure 2a, b shows the spatial distribution of calcium and magnesium in the study area.
Sodium and potassium
The concentration of sodium ions (Na+) in the collected groundwater samples varies from 18 to 560 mg/l (Table 1). The maximum permissible limit of sodium is 200 mg/l and the present study reveals that few samples exceed the permissible limit of WHO and ISI. Groundwater with high Na+ content is not suitable for agricultural use, as it tends to deteriorate the soil. Potassium is a naturally occurring element; however, its concentration remains quite lower compared with Ca2+, Mg2+ and Na+. Its concentration in drinking water seldom reaches 20 mg/l (Hounslow 1995). The concentration of K+ is between 0.5 and 83 mg/l in the groundwater of the study area. The maximum permissible limit of potassium in drinking water is 12 mg/l, and it was found that few samples exceeded the permissible limit of WHO. The higher concentration of potassium in groundwater is due to the anthropogenic sources and saline water intrusion (Krishnakumar et al. 2009). Figure 2c, d shows the spatial variation of sodium and potassium in the study area.
Chloride
Chloride ion (Cl−) concentration in the collected groundwater samples varies from 21.3 to 756.2 mg/l with a mean value of 169.7 mg/l (Table 1). According to the WHO (2011) standards, the maximum permissible limit of chloride in the groundwater is 600 mg/l. The increased chloride concentration in a freshwater aquifer is one of the indicators of seawater intrusion. Appelo and postma (1993) and Raju et al. (2011) have suggested that the high concentration of chloride may also result from pollution by domestic sewage waste, sand leaching and saline residues in soil.
Based on the ionic concentration, sodium and chloride are found to be the dominant cation and anion, respectively. Mostly, sodium and chloride dominate the seawater ionic content, while calcium and bicarbonate are generally the major ions of freshwater (Hem 1985). Therefore, the higher concentration of sodium and chloride ions in the groundwater from the study area indicates the significant effect of saltwater intrusion in few regions. The spatial distribution of chloride in the study area is shown in Fig. 3a.
a Spatial distribution of chlorine (Cl−) in the study area. b Spatial distribution of bicarbonate (\({\text{HCO}}_{3}^{ - }\)) in the study area. c Spatial distribution of sulfate (\({\text{SO}}_{4}^{2 - }\)) in the study area. d Spatial distribution of total dissolved solids (TDS) in the study area
Bicarbonate
The observed \({\text{HCO}}_{3}^{ - }\) values in the groundwater samples range from 110 to 549 mg/l (Table 1) Bicarbonate is the dominant anion in the majority of the samples, except in few groundwater samples collected near the coast. The higher concentration of \({\text{HCO}}_{3}^{ - }\) in the water shows the dominance of mineral dissolution (Stumm and Morgan 1996). The spatial distribution of the bicarbonate ion in the study area is shown in Fig. 3b.
Sulfate
The concentration of sulfate (\({\text{SO}}_{4}^{2 - }\)) ranges from 2 to 78.5 mg/l with an average value of 34.3 mg/l (Table 1). High value of sulfate content in the groundwater is due to reduction, precipitation, solution and concentration of the traverse through the sedimentary rock such as gypsum and anhydrite (Hounslow 1995). In the study area, the sulfate concentration is within the limit of WHO and ISI standards. The spatial distribution of sulfate ions in the study area is given in Fig. 3c.
Total dissolved solids (TDS)
According to WHO specifications, TDS in groundwater up to 500 mg/l is the highest desirable and up to 1000 mg/l is maximum permissible. In the study area, the TDS values vary between a minimum of 41.6 mg/l and a maximum of 1775 mg/l (Table 1). According to the Davis and De wiest (1966) classification of groundwater samples based on TDS, 60 % of the total groundwater samples in the study area are desirable for drinking (TDS < 500 mg/l), 23.3 % of samples are permissible for drinking (500–1000 mg/l) and 16.6 % are suitable for irrigation purposes. The spatial distribution of TDS in the study area is given in the Fig. 3d.
Hydrogeochemical facies
The geochemical evolution of groundwater can be understood by plotting the concentrations of major cations and anions in the piper trilinear diagram (Piper 1944). The nature and distribution of hydrochemical facies can be determined by providing insights into how groundwater quality changes within and between aquifers. Trilinear diagrams can be used to delineate the hydrogeochemical facies, because they graphically demonstrate the relationships between the most important dissolved constituents in a set of groundwater samples. The hydrogeochemistry of groundwater in the study area was evaluated using the concentrations of major cations (Ca2+, Mg2+, Na+ and K+) and anions (\({\text{HCO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\) and Cl−) in meq/l. The hydrogeochemical facies for groundwater in the study area is shown in a piper diagram (Fig. 4). The piper diagram indicates that sodium is the major cation and chloride is the major anion.
Pulido-Leboeuf (2004) pointed out that the groundwater of Na–Cl type will generally influence a strong seawater intrusion; anthropogenic contamination is caused by excessive use of fertilizers in the coastal land. In some regions, calcium- and magnesium-based water type is seen. The sources of calcium and magnesium in these regions are probably the shell deposits of fluvio-marine origin (Hounslow 1995).
Sodium percentage
The sodium in irrigation waters is usually denoted as percent sodium or sodium percentage. According to Wilcox (1955), for all natural waters Na % is a common parameter to assess its suitability for irrigational purposes. The sodium percent (Na %) values are obtained using the following equation:
where all ionic concentrations are expressed in meq/l.
The groundwater samples according to Wilcox classification (Table 2) on the basis of sodium percent shows that 53.33 % of the groundwater samples are of uncertain quality for irrigation, 16 % are within the permissible limit, 30 % are under good quality limit and no samples are in the unsuitable category.
Sodium absorption ratio
Sodium absorption ratio (SAR) is a measure of the suitability of groundwater for irrigation usage, because sodium concentration can reduce the soil permeability and soil structure (Todd 2007). SAR is a measure of alkali/sodium hazard to crops and is estimated by the following formula:
where the concentrations of sodium, calcium and magnesium are in meq/l.
The SAR value of water for irrigation purposes has a significant relationship with the extent to which sodium is absorbed by the soils. Irrigation using water with high SAR values may require soil amendments to prevent long-term damage to the soil, because the sodium in the water can displace the calcium and magnesium in the soil. This will cause a decrease in the ability of the soil to form stable aggregates and loss of soil structure. This will also lead to a decrease in infiltration and permeability of the soil to water, leading to problems with crop production (Chandrasekar et al. 2013). The calculated values of SAR in the study area vary between 1.55 and 11.18 meq/l. The classification of groundwater samples based on SAR values are given in Table 2. The SAR values of the majority of the samples are found within the range of excellent to good category, except sample nos 1, 25 and 26 which were found to be unsuitable for irrigation purpose.
USSL plot
The United States Salinity Laboratory (USSL) has constructed a diagram for the classification of irrigation waters (Wilcox 1955) describing 16 classes with reference to SAR as index for sodium hazard and EC as an index for salinity hazard. The USSL diagram highlights that 33 % of the samples fall under the field of C3S1, which indicates water having high salinity and low sodium alkali hazard (Fig. 5). Three of the samples fall under the C4S3, C3S3 and C3S2 category, respectively, indicating high to very high salinity hazard and medium to high sodium hazard, while the remaining samples fall under the C2S1 and C1S1 regions indicating low and medium saline and sodium hazards.
Wilcox diagram
Wilcox (1955) classified groundwater for irrigation purposes by correlating percent sodium and EC, which suggests that 60 % of the total of 30 samples fall under excellent to good limit and 30 % fall under good to permissible limit (Fig. 6). Only 10 % of the samples (W1, W25, W26) fall under the doubtful to unsuitable limit, which illustrates that the groundwater from these wells are not fit for agricultural usages.
Mechanisms controlling the groundwater chemistry
Reactions between aquifer minerals and groundwater have a significant role on water quality, which are also useful to understand the genesis of groundwater (Cederstorm 1946). Commonly, groundwater chemistry in the study region is controlled by different processes and mechanisms. Gibbs plot is used here to understand and differentiate the influences of rock–water interaction, evaporation and precipitation on water chemistry (Gibbs 1970). The Gibbs diagram (Fig. 7a, b) illustrates that most of the groundwater samples from the study area falls under the rock-dominant region. The cation versus TDS curves denote that the cations in some of the wells may be derived from evaporation or crystallization processes. The Gibbs diagrams suggests that the weathering of rocks primarily controls the major ion chemistry of groundwater in this region.
Conclusions
Contamination of groundwater generally results in poor drinking water quality, loss of water supply, high cleanup costs, high-cost alternative water supplies and potential health problem. In the present study, the interpretation of hydrochemical analysis reveals that the groundwater in the study area is slightly acidic in nature. The sequence of the abundance of the major cations is Na+ > Ca2+ > Mg2+ > K+ and anions is \({\text{Cl}}^{ - } > {\text{HCO}}_{3}^{ - } > {\text{SO}}_{4}^{2 - }\). The dominant cation and anion are sodium and chloride respectively, which shows the saline nature of the groundwater in some regions. In the study area, rock weathering and evaporation processes are the major hydrogeochemical processes responsible for the concentration of major ions in groundwater. The drinking water quality analysis shows that groundwater from the wells W25 and W26 are not fit for drinking, as they have higher concentration of ions than the standard permissible limits. The groundwater classification based on their suitability for irrigation also reveals that the wells W1, W25 and W26 are unsuitable for irrigation. So, among the 30 well samples analyzed, the groundwater from the wells W1, W25, W26 were found to be more hazardous in the study area. The harmful nature of groundwater may be due to natural saline water intrusion and also because of anthropogenic activities.
References
APHA (1995) Standard methods for the examination of water and waste water, 19th edn. American Public Health Association, Washington, DC
Appelo CAJ, Postma D (1993) Geochemistry, groundwater and pollution. AA Balkema, Rotterdam
Babiker IS, Mohamed MAA, Hiyama T (2007) Assessing groundwater quality using GIS. Water Resour Manag 21:699–715
Bouwer H (1978) Ground water hydrology. McGraw-Hill, New York
Burroughs PA, McDonnell RA (1998) Principles of geographical information systems. Oxford University Press, Oxford, p 333
Cederstorm DJ (1946) Genesis of groundwater in the coastal plain of Virginia. Environ Geol 41:218–245
CGWB (2008) District groundwater Brochure, Kanyakumari district, Tamilnadu. Government of India, Ministry of Resources
Chandrasekar N, Selvakumar S, Srinivas Y, John Wilson JS, Simon Peter T, Magesh NS (2013) Hydrogeochemical assessment of groundwater quality along the coastal aquifers of southern Tamil Nadu, India. J Environ Earth Sci 71(11):4739–4750. doi:10.1007/s12665-013-2864-3
Davis SN, De Wiest RJM (1966) Hydrogeology, vol 463. Wiley, New York
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Sci J 170:795–840
GSI (1995) Geological survey of India, geological and mineral map of Tamil Nadu and Pondicherry (scale 1:500000)
Hem JD (1985) Study and interpretation of the chemical characteristics of natural waters, 3rd edn. USGS Water Supply Paper. 2254:117–120
Hounslow AW (1995) Water quality data—analysis and interpretation. Lewis Publishers, Boca Raton
Indian Standard Institution (ISI) (1983) Drinking water standard substances or characteristic affecting the acceptability of water for domestic use. ISI0500, pp 1–22
Jacob CT, Azariah J, Viji Roy AG (1999) Impact of textile industries on river Noyyal and riverine groundwater quality of Tirupur, India. Pollut Res 18(4):359–368
Jaiswal RK, Mukherjee S, Krishnamurthy J, Saxena R (2003) Role of remote sensing and GIS—techniques for generation of groundwater prospect zones towards rural development–an approach. Int J Remote Sens 24(5):993–1008
Krishnakumar S, Ram Mohan V, Dajkumar SJ, Jeevanandam M (2009) Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ Monit Assess 159(1–4):341–351
Nickson R, McArthur JM, Shrestha B, Kyaw-Myint TO, Lowry D (2005) Arsenic and other drinking water quality issues, Muzaffargarh district, Pakistan. Appl Geochem 20:55–68
Pawar NJ, Shaikh IJ (1995) Nitrate pollution of ground waters from shallow basaltic aquifers, Deccan trap Hydrologic Province, India. Environ Geol 25:197–204
Piper AM (1944) A graphic procedure in geochemical interpretation of water analysis. Trans Am Geophys Union 25(6):914–928
Pulido-Leboeuf P (2004) Seawater intrusion and associated processes in a small coastal complex aquifer (Castell deFerro, Spain). Appl Geochem 19:1517–1527
Raju NJ, Shukla UK, Ram P (2011) Hydrogeochemistry for the assessment of groundwater quality in Varanasi: a fast-urbanizing center in Uttar Pradesh, India. Environ Monit Assess 173:279–300
Sarath Prasanth SV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha district. Appl Water Sci, Kerala, India. doi:10.1007/s13201-012-0042-5
Srinivas Y, Hudson Oliver D, Stanley Raj A, Chandrasekar N (2013) Evaluation of groundwater quality in and around Nagercoil town, Tamilnadu, India: an integrated geochemical and GIS approach. J Appl Water Sci 3:631–651
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley, New York, p 1022
Sujatha D, Reddy RB (2003) Quality characterization of groundwater in the south eastern parts of the Ranga Reddy district, Andhra Pradesh, India. Environ Geol 44(5):570–576
Todd DK (2007) Groundwater hydrology, 3rd edn. Wiley, New York, p 347
Wilcox LV (1955) Classification and use of irrigation waters, USDA Circular No. 969, p 19
World Health Organization (WHO) (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
Acknowledgments
The present study was carried out under the UGC major research project. The first author is grateful to the UGC for providing the financial support under this major research project No. 41-940/2012 (SR). The authors are very much thankful to the anonymous reviewers for their critical review and comments that helped immensely in improving the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Srinivas, Y., Aghil, T.B., Hudson Oliver, D. et al. Hydrochemical characteristics and quality assessment of groundwater along the Manavalakurichi coast, Tamil Nadu, India. Appl Water Sci 7, 1429–1438 (2017). https://doi.org/10.1007/s13201-015-0325-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0325-8