Abstract
In this research, a strong anionic resin was prepared to remove chloride ions. This study was performed in a laboratory pilot to evaluate effective parameters such as temperature, amine flow rate, pH, chloride ion concentration and concentration of resin caustic. The purpose of this work is to determine kinetics of the resin reaction and determination of the optimal operating conditions in order to achieve maximum saturation. This study shows that volume of distilled water passing through resin to eliminate alkalinity at temperatures 40 °C, 50 °C and 60 °C is equal to 5500 ml, 5000 ml and 7000 ml, respectively. This work states that a temperature 50 °C is more appropriate than other temperatures. The results of this research show that volume of caustic for resin recovery is 8.5 L at a temperature of 50 °C. This study shows that amount of distilled water to eliminate alkalinity of the resin is equal to 5 L. Results show that the order of reaction to chloride ion concentration is equal to 0.794 and reaction constant is equal to exp (−1.8753).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The consequences of the presence of salt in the amine solution are amine contamination, followed by reduced efficiency of the operating unit (Omer et al. 2022). Increasing electrical conductivity of solution accelerates the electrochemical reactions, including corrosion reactions (Verma et al. 2022). The total concentration of heat stable salt in amine should not exceed 0.5 wt.% (Çevirim-Papaioannou et al. 2022). One way to release contaminated amines with heat stable salts is to add strong bases such as calcium hydroxide or potassium carbonate to the solution (Aryal et al. 2022). This does not affect anion content of the heat stable salts, but forms sodium salts instead of amine salts (Yang et al. 2022a). Therefore, it seems necessary to create a de-chlorination unit and a heat stable salt separation unit next to the gas sweetening unit for amine recovery (Yang et al. 2022b; Jeyaseelan et al. 2022). The anions of the salts in gas enter to the amine and form a heat stable salt. For this reason, presence of high chloride ions and heat stable salts has been observed in some refineries in recent years (Yuan et al. 2022). The anionic compounds are separated using resin to solve this problem (Shi et al. 2022). Increasing efficiency of gas sweetening process and quality of amine is always one of the most basic operational plans of refineries (Abuzalat et al. 2022; Zhang et al. 2022; Zhao et al. 2022).
In this study, a strong anionic resin was prepared to remove chloride ions. This research was performed in a laboratory pilot to study parameters such as operating temperature, amine flow rate, pH, chloride ion concentration and concentration of caustic. The purpose of this research is to determine kinetics of the reaction of resin with caustic solution.
Materials and method
A study of following items is necessary in order to conduct this research: (A) Determination of synthetic reaction. (B) Calculation of resin capacity. (C) Calculation of break through time (BTT) of the resin. (D) Determination of consumed water to neutralize alkaline effect of caustic. (E) Determination of functional and optimal pH of chlorine adsorption process. (F) Determination of optimal temperature of amine recycling and resin recovery. (G) Determination of caustic consumption to recovery each liter of resin.
Required chemicals
The following chemicals are used in this study: The Diethanolamine 30%, the nitric acid 65%, hydrochloric acid 37%, silver nitrate tetrazole, sodium hydroxide 99%, phenolphthalein, lead acetate paper, standard solution in acidic medium, standard solution in neutral medium, standard solution in alkaline medium, standard chloride solution 1000 mg/l. All of the solutions are provided by the Merck Company, Germany.
Special properties of M-500 as anionic resin
Table 1 presents main specifications of the resin. In addition, Table 2 shows physical and chemical properties of the resin.
Results and discussion
Investigation of distilled water at temperatures 40 °C, 50 °C and 60 °C
The resin becomes alkaline due to that the pH of diethanolamine and 4% caustic is in the alkaline range. The alkalinity of resin must be eliminated in order to perform saturation and recovering steps at temperatures 40 °C, 50 °C and 60 °C. Deionized water is used to neutralize the alkalinity of the resin. This study shows that volume of deionized water passing through resin at temperatures of 40 °C, 50 °C and 60 °C is equal to 5500, 5000 and 7000 ml, respectively. Table 3 shows volume of distilled water and caustic at temperatures 40 °C, 50 °C and 60 °C.
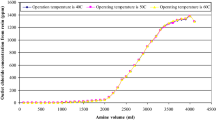
Table 3 shows that amount of caustic to recover resin is 8.5 L at a temperature of 50 °C. Also, the amount of distilled water to eliminate alkalinity of resin is equal to 5 L. Table 3 states that a temperature 50 °C is more appropriate than other temperatures. Fig. 1 shows concentration of chloride leaving the resin in terms of volume of amine at temperatures 40 °C, 50 °C and 60 °C.
The steps of recovery and elimination of resin alkalinity in terms of caustic and distilled water at temperatures 40 °C, 50 °C and 60 °C are shown in Fig. 2. Fig. 2 shows that the amount of caustic and water becomes minimum at a temperature of 50 °C.
The amount of caustic passing through resin in terms of chloride ion concentration at temperatures 40 °C, 50 °C and 60 °C is shown in Fig. 3.
The saturation time of resin in terms of chloride ion concentration at temperatures 40 °C, 50 °C and 60 °C is shown in Fig. 4. Fig. 4 shows that the chlorine ion concentration increases at first and then, decreases. The equation in Fig. 4 shows the changes of chloride ion concentration in terms of time. The regression of this equation is equal to 0.9926.
Determination of reaction kinetics
After measuring chloride concentration at temperatures 40 °C, 50 °C and 60 °C, it was found that ion exchange reaction was a quasi-first order. Equation of this reaction is \(- r_{{\text{A}}} = kC_{{\text{A}}}^{{\text{n}}}\), and k is called reaction rate constant. Also, parameter n is presented as degree of reaction. The Eq. 2 was used to calculate reaction order and reaction constant. At the first, a 0.5 wt.% caustic is made, and then, this solution and diethanolamine are heated to a temperature 50 °C. This step is opposite of previous steps, exactly. In other words, first, a 0.5 wt.% caustic solution is passed through the resin to recovery it and then, a diethanolamine solution containing 1400 ppm of chloride ion is passed through resin bed to saturate the resin. The chloride ion measuring testing is performed after 100 ml of caustic and amine passes through the resin. Finally, the Eqs. 1 and 2 are used to calculate values of order and constant of reaction.
According to the chemical equation of reaction, ln is taken from the sides of equation. According to the laboratory results, the diagram for Eq. 2 is drawn. The constant reaction parameters and reaction order are obtained, finally. Table 4 presents laboratory results for determination of reaction constant and reaction order with respect to the chloride ion concentration.
Figure 5 shows chemical equation of reaction of chloride ion. The equation in Fig. 5 shows the logarithmic of chemical reaction in terms of chloride ion concentration. The regression of this equation is equal to 0.9942.
According to Fig. 5, it is shown that reaction order to chloride ion is equal to 0.794, and reaction constant is equal to exp (−1.8753).
Conclusion
The laboratory results show that volume of amine for resin saturation at temperatures 40 °C, 50 °C and 60 °C is equal to 3000 ml. This work shows that resin will be recovered after passing 9500, 8500 and 10,000 ml of 4% caustic solution at temperatures 40 °C, 50 °C and 60 °C, respectively. This research shows that volume of deionized water to eliminate alkalinity at temperatures 40 °C, 50 °C and 60 °C is equal to 5500, 5000 and 7000 ml, respectively. This work shows that a temperature 50 °C is more appropriate than other temperatures. In addition, the amount of caustic to recover resin is 8.5 L at a temperature 50 °C. Also, amount of distilled water to eliminate alkalinity of resin is equal to 5 L. This study shows that the reaction order to chloride ion is equal to 0.794, and reaction constant is equal to exp (−1.8753).
Data availability
Not applicable.
Code availability
Not applicable.
References
Abuzalat O, Tantawy H, Mokhtar M, Baraka A (2022) Nano-porous bimetallic organic frameworks (Fe/Co)–BDC, a breathing MOF for rapid and capacitive removal of Cr-oxyanions from water. J Water Process Eng 46:102537
Aryal RL, Thapa A, Poudel BR, Pokhrel MR, Dahal B, Paudyal H, Ghimire KN (2022) Effective biosorption of arsenic from water using La(III) loaded carboxyl functionalized watermelon rind. Arab J Chem 15(3):103674
Çevirim-Papaioannou N, Jo Y, Franke K, Fuss M, Blochouse B, Altmaier M, Gaona X (2022) Uptake of niobium by cement systems relevant for nuclear waste disposal: impact of ISA and chloride. Cem Concr Res 153:106690
Jeyaseelan A, Viswanathan N, Naushad M, Albadarin AB (2022) Eco-friendly design of functionalized graphene oxide incorporated alginate beads for selective fluoride retention. Diam Relat Mater 121:108747
Omer AM, Dey R, Eltaweil AS, Abd El-Monaem EM, Ziora ZM (2022) Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab J Chem 15(2):103543
Shi Y, Cheng X, Wan D, Chen X, Liu Y, Zhang Z, Wang Y, Gao Y (2022) Construction of the micro-electrolysis system by Fe0 and clay-carbon derived from oil refining for the removal of ozone disinfection by-products. Colloids Surf A 637:128224
Verma R, Maji PK, Sarkar S (2022) Synthesis and validation of polystyrene-based polyethylenimine composite for Cr(VI) removal from aqueous solution: performance and mechanism. J Environ Chem Eng 10(1):107119
Yang X, Hsia T, Merenda A, Riyadh ALA, Dumee LF, Thang SH, Kong L (2022a) Constructing novel nanofibrous polyacrylonitrile (PAN)-based anion exchange membrane adsorber for protein separation. Sep Purif Technol 285:120364
Yang J, Shi K, Wu F, Tong J, Su Y, Liu T, He J, Mocilac P, Hou X, Wu W, Shi W (2022b) Technetium-99 decontamination from radioactive wastewater by modified bentonite: batch, column experiment and mechanism investigation. Chem Eng J 428:131333
Yuan Y, Garg S, Wang Y, Li W, Chen G, Gao M, Zhong J, Wang J, Waite TD (2022) Influence of salinity on the heterogeneous catalytic ozonation process: implications to treatment of high salinity wastewater. J Hazard Mater 423:127255
Zhang Y, Liu Q, Ma W, Liu H, Zhu J, Wang L, Pei H, Liu Q, Yao J (2022) Insight into the synergistic adsorption-reduction character of chromium(VI) onto poly(pyrogallol-tetraethylene pentamine) microsphere in synthetic wastewater. J Colloid Interface Sci 609:825–837
Zhao Y, Zhao C, Yang Y, Li Z, Qiu X, Gao J, Ji M (2022) Adsorption of sulfamethoxazole on polypyrrole decorated volcanics over a wide pH range: mechanisms and site energy distribution consideration. Sep Purif Technol 283:120165
Acknowledgements
This research did not receive any specific funding.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farahbod, F. Experimental study of order and constant rate of chlorine removal reaction using ion exchange resin. Appl Water Sci 13, 167 (2023). https://doi.org/10.1007/s13201-023-01969-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01969-4