Abstract
Neutral leaching or water washing is used for Cl− and partially Ca2+ ion removal in order to save the leaching reagents for the next steps in hydrometallurgical treatment of electric arc furnace dust. This pre-treatment of the material leads to the generation of strong alkaline (pH = 11.9–12.7) leachate (wastewater), which underlies precipitation of Ca and other accompanying metals. This work presents results from the study of various economically differing (cost and time) techniques for elimination of these disadvantageous phenomena. The aim of the experimental study was to obtain water suitable for reuse in the leaching or for discharge into a recipient in accordance with valid legislation. The experiments were focused on the removal of precipitates, or metals which create the precipitates, and pH decreasing. Our results indicate that five-minute agitation of 4 g solid NaHCO3 and 1 L of neutral leachate and subsequent ion exchange in the sequence of strong-acid cation and strong-base anion exchange treatment led to the acquisition of water without creation of precipitates, with pH below 8.5 and conductivity approx. 0.03 mS. The removal of Cl− ions was not complete.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is possible to use electric arc furnace (EAF) dust as a secondary raw material in the production of mainly Zn, Fe and Pb (Oustadakis et al. 2010). Besides these elements, EAF dust contains predominantly Ca and chlorides, and minor components which are most often Na, Mg and Cd (Montenegro et al. 2013). There are three methods of EAF dust processing: pyrometallurgical, hydrometallurgical (Oustadakis et al. 2010; Raza et al. 2016), and their pyro-hydrometallurgical combination (thermal reduction followed by leaching) (Kukurugya et al. 2015; Chairaksa-Fujimoto et al. 2016).
The water leaching/washing procedure is usually used as a pre-treatment step in hydrometallurgical processing of EAF dust with the aim of removing water-soluble chlorides of Na and K (Chen et al. 2011; Wang et al. 2016) and also as much Ca as possible in order to prevent overconsumption of acid leaching reagents in the next leaching step. Ca is leachable in the pH range 6.5–11.0 according to the reaction:
The suitable equilibrium pH for water leachate is from 9.0 to 11.0, preferably around 10.0. If the water leachate pH is higher than 11.0 and OH− ions are present, Ca starts precipitating as hydroxide, part of Pb dissolves from the dust in soluble species (Montenegro et al. 2016), and additionally, there may be partial extraction of Zn from ZnO (the majority phase of EAF dust), which is soluble in the created alkaline solution (Kukurugya et al. 2015; Chairaksa-Fujimoto et al. 2016).
Various methods exist for removal of metals from wastewater, including physicochemical processes (precipitation, ion exchange), adsorption, electrochemical treatment (electrocoagulation, elector-floatation, and electrodeposition), reverse osmosis, membrane filtration (Fu and Wang 2011; Abdelwahab et al. 2013; Azimi et al. 2017), and also utilization of new materials and technique as synthetic polymer membrane for adsorption (Ibrahim et al. 2018) or ultrafiltration (Nayak et al. 2020). The chemical precipitation is most widely practiced in conventional treatment of industrial waters, mainly for the simplicity of the process control, low-cost operation and effectivity in a wide range of temperature (Benalia et al. 2022). Commonly used inorganic precipitants for metal precipitation are natrium hydroxide/caustic soda (NaOH), calcium hydroxide/lime (Ca(OH)2), soda ash (Na2CO3), sodium bicarbonate (Na(HCO3)2), sodium sulfide (Na2S) and sodium hydrosulfide (NaHS). For chemical precipitation of metals from water is very important, the adjustment of the pH and basic conditions with pH = 11 are preferred (Azimi et al. 2017). Chemical precipitation with NaOH/Ca(OH)2 is one of the low-cost and relatively simple ways of heavy metal removal from wastewater (Aziz et al. 2008). Lime (CaO/Ca(OH)2) may be used to precipitate soluble metals into insoluble hydroxide forms in an alkaline environment.
However, hydroxide precipitation also has some limitations: (I) production of secondary waste in the form of relatively low-density metal hydroxide sludge, (II) creation of amphoteric hydroxides from some metals, (III) the ideal pH for high stability of some metal hydroxides may put other metals back into solution, (IV) the presence of complexing agents in the wastewater may lead to inhibition of hydroxide precipitation (Fu and Wang 2011; Oncel et al. 2013). For Ca and Mg removal by precipitation, it is also possible to use Na2CO3 or NaHCO3, which may create carbonates according to reactions (Eqs. 2 and 3) (Maccagni 2016):
The removal of ions created in a given water environment by precipitation (the primary method of wastewater treatment) is very often combined with ion exchange as a secondary treatment method (Fu and Wang 2011; Victor-Ortega et al. 2016; Bashir et al. 2018). Ion exchange, which comprises anion and cation exchange, is very often used for water treatment (Indarawis and Boyer 2013). Ion exchange technology possesses a series of characteristics which make it very attractive, i.e., simplicity, effectiveness, selectivity, recovery, relatively low cost (Victor-Ortega et al. 2015), high-treatment capacity, removal efficiency, fast kinetics (Fu and Wang 2011), treatment of a large volume of effluent at one time (Shaidan et al. 2012) and easy recovery and reuse in generation operations (Lee et al. 2007; Bashir et al. 2018). In this method, ions are removed through their transfer to a solid material, usually resin. Ion exchange methods are widely utilized for removal of heavy metals (Abdelwahab et al. 2013; Barakat 2011; Lee et al. 2007; Alyüz and Veli 2009) from water or industrial wastewater, hardness (Apell and Boyer 2009) or Ca2+ (Yu et al. 2015), and other ions from natural water (Indarawis and Boyer 2013), high salinity (Na+ and Cl− ions) from wastewater after its primary treatment (Victor-Ortega et al. 2016, 2015), chloride (Lv et al. 2009), fluoride, nitrate (Milmile et al. 2011) and sulphate ions (Milmile et al. 2011; Dron and Dodi 2011). The affinity of anion species to strong-base anion exchange resin increases in the order OH− < HCO3− < Cl− < NO3− < SO42− (Dron and Dodi 2011). Hydrogen ions released from the cation exchanger in the H+ cycle to a strong alkaline environment can neutralize OH− ions after absorption of all metal ions into the ion exchange resin (Lee et al. 2007).
The aim of the presented work was to remove metals or their precipitates and to decrease pH of EAF dust water leachate in order to obtain water suitable for reuse in the other leaching or for discharge into a recipient in accordance with Slovak valid legislation. The specific objectives of the work were: (1) to find the most advisable precipitation procedure from the economic (cost and time) and removal efficiency points of view; and (2) to compare and evaluate removal efficiencies for anion–cation and cation–anion sequences of ion exchange applied after the most advisable precipitation procedure.
Experimental
Material
EAF dust material from the company Železiarne Podbrezová, a.s. (Slovakia) was used for water leaching procedures. The leachate, or more specifically its filtrate, obtained by leaching of 100 g (or 50 g) dust with 1.0 L (or 0.5 L) of distilled water (solid–liquid ratio of 1:10) under atmospheric pressure and constant speed of stirring (500 rpm) for a period of one hour, was prepared before each treatment experiment.
Ion exchange resins and reagents
Commercial strong-acid cation exchanger Amberlite IR120 (Rohm and Haas) and strong-base anion exchanger Amberlite IRA 410 (Rohm and Haas) were used for the ion exchange experiments. The principal physical and chemical properties of these ion exchangers are listed in Table 1.
Before utilization of the ion exchanger, 100 g of the resin was saturated in ultrapure water, introduced into a glass column and conditioned/regenerated to the given cycles with 2 M HCl solution for the cation exchanger in the H+ cycle and 1 M NaOH solution for the anion exchanger in the OH− cycle. Analytical grade concentrated HCl and NaOH and ultrapure water were used for preparation of the solutions. The ultrapure water was prepared using an ELGA LabWater deionizer.
Precipitation and ion exchange methods
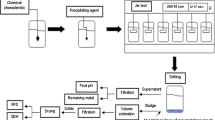
With the aim of removing metals forming precipitates, three methods were applied in order of increasing cost and decreasing time requirements:
Spontaneous precipitation—the effect of time (3 and 7 days) on the spontaneous removal of metals from 870 mL of filtered leachate was monitored.
Thermal (boiling) precipitation—the effect of leachate boiling (20 min) on the removal efficiency of metals with/without cooling of suspension before filtration was monitored.
Chemical precipitation—the effects of different amounts of Na2CO3 and NaHCO3 addition and time of precipitation on the removal efficiency of metals from 100 mL of leachate were monitored. The experiments were performed by adding solid precipitants into the leachate, stirring the suspension during precipitation and then, filtering it after a given time.
Following the removal of Na+, K+ and Cl− ions from 400 mL filtrate after 5 min of chemical precipitation with NaHCO3 at concentration 4 g/L, two different ion exchange sequences were applied: anion–cation and cation–anion.
At the start of each experiment, the concentrations of the monitored elements in the leachate were determined, and at the end of experiment, their concentrations in the filtrate after precipitation and in the eluate after ion exchange were measured. The percentage removal efficiency R/% was calculated as follows:
where ci is the initial concentration of the element in the leachate before the removal experiment, and cf is the final concentration of elements in solution after this experiment in mg/L.
Analytical methods
The content of monitored elements in the leachates after neutral leaching of EAF dust, filtrates after precipitation, and eluates after ion exchanges was determined by means of high-resolution continuum source flame atomic absorption spectrometry (HR-CS FAAS) method using a contrAA700 spectrometer (Analytik Jena AG). The most suitable working conditions for analysis (gas flow and burner height) had been experimentally determined prior to the analyses using a calibration solution with the highest content of a given element. Argentometric titration was used for determining the content of Cl−, and the content of HCO3− and CO32− ions was determined using acidic titration (acid neutralizing capacity, ACN to pH 4.50 and to pH 8.30). The content of SO42− was determined by means of UV/VIS absorption spectrometry on an HI 83,099 multi-parameter photometer (Hanna Instruments). For pH measurement, the Orion 410A benchtop pH/temperature meter (Thermo Electron Corporation) and, for conductivity measurement, the Orion 115A + conductivity/salinity meter (Thermo Electron Corporation) were used. Phase analysis of the solid residue after chemical precipitation was carried out on a PANanalytical X'Pert PRO MPD X-ray diffractometer using CoKα radiation (Malvern Panalytical).
Results and discussion
At first, we monitored the behavior of the leachate and EAF dust material in the process of neutral leaching and after its finalization. Some segments of washed dust after drying at 95 °C were encrusted with a deposit of white precipitates. These white precipitates were observed in the leachates, and their creation continued also after the leaching finished.
The leachates were unstable as well, considering their chemical composition. The contents of monitored components (metals and ions) in the leachates varied and their concentration ranges determined during all experiments are listed in Table 2.
As a consequence of differences in chemical composition, the conductivity also ranged from 8.43 to 9.29 mS and pH from 11.92 to 12.70.
In view of the high pH value above 11 (strong alkaline environment) and composition of the leachates, it is possible to assume that precipitates of Pb and Zn hydroxides were formed and possibly also carbonates of Ca. The solubility product constants for these elements decrease in the order Pb > Zn > Ca, and the solubility of Ca hydroxide is higher than Ca carbonate in contrast to Pb and Zn (Bobrowska-Grzesik et al. 2013). The content of these elements in the water leachate from EAF dust decreased in the order: Ca (hundreds mg/L), Pb (tens mg/L) and Zn (below ten mg/L).
Secondly, the experiments focusing on the removal of Ca, Pb and Zn by means of precipitation were carried out: spontaneous (experiments A) over 3 (A1) and 7 days (A2); thermal (experiments B) without (B1) and with cooling (B2); a combination of spontaneous and thermal (experiment C) with a sequence of spontaneous precipitation over 3 days, then thermal precipitation followed by spontaneous precipitation over 4 days; and chemical (experiments D) with Na2CO3 (D1) and with NaHCO3 (D2). The results of Ca, Pb and Zn removal efficiencies after A, B and C experiments are given in Table 3. Graphic presentation of removal efficiency of Ca, Pb and Zn after spontaneous, thermal and combined precipitation is shown in Fig. 1.
From the results of spontaneous, thermal and combined precipitation (A, B and C experiments) of metals from EAF dust water leachate, it can be concluded that the highest removal efficiency for Ca, Pb and Zn was obtained by applying the most time-consuming procedure, i.e., the combination of spontaneous and thermal precipitation.
The experiments involving chemical precipitation (D) were carried out in several steps with the aim of investigating the effect of precipitant concentrations and time of precipitation on the removal efficiency by means of:
-
increasing Na2CO3 concentration (5, 10 and 15 g/L) and constant time (30 min),
-
increasing time (5, 10, 15 and 30 min) and constant Na2CO3 concentration with the highest influence (10 g/L) in the previous step,
-
increasing NaHCO3 concentration (5, 10 and 15 g/L) and constant time (30 min),
-
increasing time (5, 10, 15 and 30 min) and constant NaHCO3 concentration with the highest influence (5 g/L) in the previous step,
-
increasing NaHCO3 concentration (1, 2 and 4 g/L) and constant time with the highest influence (5 min) in the previous step.
-
The best removal efficiencies obtained in this series of experiments with chemical precipitation (D) are shown in Table 4.
By applying Na2CO3 in the chemical precipitation, 100.0% removal efficiency of all three metals was achieved after 15 min of precipitation with the addition of 10 g precipitant to 1 L leachate. The same 100.0% removal efficiency was achieved in a shorter time (5 min) by application of NaHCO3 and with lower addition of precipitant (4 g) to 1 L leachate. The pH in the filtrate after this chemical precipitation decreased to 10.4, and the conductivity was lowest in comparison with the other experiments.
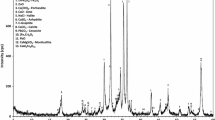
Chemical precipitation with NaHCO3 under the most appropriate conditions (time and precipitant amount) was used for consecutive evaluation of Na+, K+, Cl− and SO42− ion removal efficiency using the ion exchange method. XRD analysis of solid residue after chemical precipitation with NaHCO3 confirmed the presence of CaCO3 (Ref. Code 01-072-1937, calcium carbonate) as the majority phase in this material (Fig. 2). The presence of Pb and Zn phases, in view of their low content, was not detected. The phase of CaCO3 was also confirmed in solid residues after spontaneous and thermal precipitation.
Thirdly, the ion exchange method (E) was applied to the filtrate in the sequences cation–anion (E1) and anion–cation (E2) after chemical precipitation. In the solution after chemical precipitation, besides Na+ (from EAF dust and precipitant) and K+ (from dust) cations, there may also occur Cl− and SO42− ions (from dust), and CO32− or HCO3− ions (from dust and precipitant). The total removal efficiency after individual steps of the complete treatment of leachates, i.e., chemical precipitation (CP), anion (AE) and cation exchange (CE), along with values of pH and conductivity are shown in Table 5.
It follows from the results in Table 4 that the most efficient method of water leachate treatment is the sequence of chemical precipitation followed by cation–anion exchange. The final removal efficiencies in this sequence were: 100% for Ca2+, Pb2+, Zn2+, K+ and Cl−, 99.1% for Na+ and 58.6% for Cl− ions. The residual content of Na+ was approx. 3 mg/L and Cl− approx. 600 mg/L, conductivity ~ 0.03 mS and pH ~ 8.4 (slightly alkaline). The affinity of the monitored cations to the cation exchanger with the sulphonic functional groups (Amberlite IR 120, see Table 1) decrease in the order Pb2+ > Ca2+ > Zn2+ > K+ > Na+ (Holubicki and Kołodyńska 2012) and given the absence of Pb, Ca and Zn ions in solution after their 100% chemical precipitation, the affinity of K+ was higher than Na+ ions, which may be related to the incomplete removal of Na+ ions.
The lower removal efficiency for Cl− ions may be caused by preferred exchange of OH− ions from the anion exchanger resin for other ions in the solution with higher affinity. On the other hand, since the affinity of SO42− ions to the anion exchanger with tertiary amine functional groups (Amberlite IRA 410, see Table 1) was noticeably higher, and the affinity of HCO3− ions was slightly less than Cl− ions (Holubicki and Kołodyńska 2012), and since the removal efficiency for SO42− was 100.0%, with the greatest probability, it was SO42− ions which were responsible for the lower efficiency of Cl− ion removal. We tested this hypothesis on synthetic solutions (experiments F) containing Na+, K+, Cl− and CO32− ions (F1: 1.5 g K2CO3 + 5.0 g NaCl in 0.5 L of distilled water); Na+, K+, Cl−, CO32− and HCO3− ions (F2: 0.8 g K2CO3 + 0.7 g KHCO3 + 5.0 g NaCl in 0.5 L of distilled water); and Na+, K+, Cl−, CO32−, HCO3− and SO42− ions (F3A: 0.8 g K2CO3 + 0.7 g KHCO3 + 4.3 g NaCl + 0.5 g Na2SO4, and F3B: 0.8 g K2CO3 + 0.7 g KHCO3 + 4.3 g NaCl + 0.9 g Na2SO4 in 0.5 L of distilled water). Table 6 compares the chemical composition of the real solution treated with chemical precipitation (RSCP) and synthetic solutions (SS), conductivity and pH before and after cation–anion exchange applied to these solutions and total removal efficiencies of selected ions, which are separately in graphical form shown in Fig. 3.
The synthetic solutions contained 2.0–5.0 times more Na+, 1.8–2.4 times more K+, 4.3–4.7 times more Cl− and approx. 2.0 times more SO42− than the real solution after chemical precipitation. The removal efficiency of all ions from the synthetic solution (SSF1) was above 95.0%. Addition of HCO3− ions to the synthetic solution (SSF2) resulted in a decrease in the removal efficiency of Na+ and Cl− ions, and the addition of SO42− ions (SSF3A and SSF3B) also reduced the removal efficiency of both K+ and CO32− ions. Addition of SO42− ions to the synthetic solution in the amount approximately equal to its amount in the leachate (SSF3A) led to a marked decrease in the removal efficiency of Na+ and K+ by about 20.0% and Cl− ions by about approx. 10.0%. With the addition of double the amount of SO42− ions (SSF3B), the removal efficiency of Na+ decreased by about 20.0%, K+ about 10.0% and Cl− about 25.0% in.contrast to SSF3A. The results of these experiments confirm that the affinity of SO42−ions is higher than that of CO32− and Cl− ions. The results of the removal experiments performed on the real solution after chemical precipitation (RSCP) in comparison with the synthetic solution SSF3 suggest that increases in Na+, K+ and Cl− ion contents in the water leachate in the presence of SO42− ions may lead to a decrease in their removal efficiency.
Conclusion
This experimental study demonstrates that:
-
(1)
The most effective procedure for removal of metals creating precipitates in water leachate (Ca, Pb and Zn) is chemical precipitation using NaHCO3, which provides 100.0% removal efficiency in the shortest time and with lowest addition of reagent to the leachate. After chemical precipitation, a solution was obtained without creation of precipitates and with lower pH \(\cong\) 10. The solution treated using the proposed method contains only Na+ (from the EAF dust and the chemical reagent), K+, Cl−, SO42− (from the dust) and CO32− ions (from the reagent), which can be subsequently eliminated by means of ion exchange procedures.
-
(2)
The results of applying strong-acid cation and strong-base anion exchange to the solution after chemical precipitation show that ion exchange in the order cation–anion is more effective. The biggest shortcoming in this way of treating the EAF dust water leachate was only approx. 60.0% efficiency of Cl− ion removal, caused by presence of SO42− ions with higher affinity to the cation exchanger than Cl− ions. The results of our removal experiments carried out on the real solution after chemical precipitation in comparison with the synthetic solution suggest that increases in Na+, K+ and Cl− ion contents in the water leachate in the presence of SO42− ions may lead to a decrease in their removal efficiency.
The water leachates treated with a combination of chemical precipitation with NaHCO3, strong-acid cation and strong-base anion exchanger end up with pH below 8.5, conductivity approx. 0.03 mS and Cl− ion content approx. 340 mg/L, which however is still not in accordance with valid legislation for their discharge into a recipient (200 mg/L) (Regulation No. 269/2010).
Data availability
All data generated or analyzed during this study are presented within the submitted manuscript.
References
Abdelwahab O, Amin NK, El-Ashtoukhy E-SZ (2013) Removal of zinc ions from aqeous solution using a cation exchange resin. Chem Eng Res Des 91:165–173. https://doi.org/10.1016/j.cherd.2012.07.005
Alyüz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueos solutions by ion exchange resins. J Hazard Mater 167:482–488. https://doi.org/10.1016/j.jhazmat.2009.01.006
Apell JN, Boyer TH (2009) Combined ion exchange treatment for removal of dissolvedorganic matter and hardness. Water Res 44:2419–2430. https://doi.org/10.1016/j.watres.2010.01.004
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2017) Removal of heavy metals from industrial wastewaters: a review. Chem Bio Eng Rev 4:37–59. https://doi.org/10.1002/cben.201600010
Aziz HA, Adlan MN, Ariffin KS (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresour Technol 99:1578–1583. https://doi.org/10.1016/j.biortech.2007.04.007
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377. https://doi.org/10.1016/j.arabjc.2010.07.019
Bashir A, Malik LA, Ahad S, Manzoor T, Bhat MA, Dar GN, Pandith AH (2018) Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ Chem Lett 17:729–754. https://doi.org/10.1007/s10311-018-00828-y
Benalia MCh, Youcef L, Bouaziz MG, Achour S, Menasra H (2022) Removal of heavy metals from industrial wastewater by chemical precipitation: mechanisms and sludge characterization. Arab J Sci Eng 47:5587–5599. https://doi.org/10.1007/s13369-021-05525-7
Bobrowska-Grzesik E et al (2013) Chemical Elements Compendium. Ing. Václav Helán – 2THETA, Český Těšín
Chairaksa-Fujimoto R, Maruyama K, Miki T, Nagasaka T (2016) The selective leaching of zinc oxide from electric arc furnace dust pre-treated with calcium oxide. Hydrometallurgy 159:120–125. https://doi.org/10.1016/j.hydromet.2015.11.009
Chen WS, Shen YH, Tsai MS, Chang FC (2011) Removal of chloride from electric arc furnace dust. J Hazard Mater 190:639–644. https://doi.org/10.1016/j.jhazmat.2011.03.096
Dron J, Dodi A (2011) Comparison of adsorption equilibrium models for the study of Cl-, NO3- and SO42- removal from aqueous solutions by anion exchange resin. J Hazard Mater 190:300–307. https://doi.org/10.1016/j.jhazmat.2011.03.049
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters. A Review J Environ Manage 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Holubicki Z, Kołodyńska D (2012) Selective removal of heavy metals ions from waters and waste waters using ion exchange methods. Chapter 8. Open Access book publisher: intech; 2012 193–240. https://doi.org/10.5772/51040
Ibrahim GPS, Isloor AM, Inamuddin AAM, Ismail AF, Kumar R, Ahamed MI (2018) Performance intensification of the polysulfone ultrafiltration membrane by blending with copolymer encompassing novel derivative of poly(styrene-co-maleic anhydride) for heavy metal removal from wastewater. Chem Eng J 353:425–435. https://doi.org/10.1016/j.cej.2018.07.098
Indarawis KA, Boyer TH (2013) Superposition of anion and cation exchange for removal of natural water ions. Sep Purif Technol 118:112–119. https://doi.org/10.1016/j.seppur.2013.06.044
Kukurugya F, Vindt T, Havlík T (2015) Behaviour of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions. Hydrometallurgy 154:20–32. https://doi.org/10.1016/j.hydromet.2015.03.008
Lee I-H, Kuan Y-C, Chern J-M (2007) Equilibrium and kinetics of heavy metal ion exchange. J Chin Inst Chem Eng 38:71–84. https://doi.org/10.1016/j.jcice.2006.11.001
Lv L, Sun P, Gu Z, Du H, Pang X, Tao X, Xu R, Xu L (2009) Removal of chloride ion from aqueos solution by ZnAl-NO3 layered double hydroxides as anion-exchanger. J Hazard Mater 161:1444–1449. https://doi.org/10.1016/j.jhazmat.2008.04.114
Maccagni MG (2016) INDUTEC®/EZINEX® Integrate Process on Secondary Zinc-Bearing Materials. J Sustain Metall 2:133–140. https://doi.org/10.1007/s40831-016-0041-0
Milmile SN, Pande JV, Karmakar S, Bansiwal A, Chakrabarti T, Biniwale RB (2011) Equilibrium isotherm and kinetic modeling of the absorption of nitrates by anion exchange Indion NSSR resin. Desalination 276:38–44. https://doi.org/10.1016/j.desal.2011.03.015
Montenegro V, Oustadakis P, Tsakiridis PE, Agatzini-Leonardou S (2013) Hydrometallurgical Treatment of Steelmaking Electric Arc Furnace Dusts (EAFD). Metall Mater Trans B 44:1058–1069. https://doi.org/10.1007/s11663-013-9874-0
Montenegro V, Agatzini-Leonardou S, Oustadakis P, Tsakiridis P (2016) Hydrometallurgical Treatment of EAF dust by Direct Sulphuric Acid Leaching at Atmospheric Pressure. Waste Biomass Valor 7:1531–1548. https://doi.org/10.1007/s12649-016-9543-z
Nayak MCh, Isloor AM, Inamuddin LB, Marwani HM, Khan I (2020) Polyphenylsulfone/multiwalled carbon nanotubes mixed ultrafiltration membranes: fabrication, characterization and removal of heavy metals Pb2+, Hg2+, and Cd2+ from aqueous solutions. Arab J Chem 13:4661–4672. https://doi.org/10.1016/j.arabjc.2019.10.007
Oncel MS, Muhcu A, Demirbas E, Kobya M (2013) A comparative study of chemical precipitation and electrocoagulation for treatment of coal acid drainage wastewater. J Environ Chem Eng 1:989–995. https://doi.org/10.1016/j.jece.2013.08.008
Oustadakis P, Tsakiridis PE, Katsiapi A, Agatzini-Leonardou S (2010) Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). J Hazard Mater 179:1–7. https://doi.org/10.1016/j.jhazmat.2010.01.059
Raza N, Raza W, Zafar ZI, Kumar RV (2016) Benefication of Zinc from Electric Arc Furnace Dust Using Hydrometallurgical Approach. Russ J Appl Chem 89(5):836–845. https://doi.org/10.1134/S1070427216050244
Regulation of the Government of the Slovak Republic No. 269/2010: quality objectives of surface waters and on limit values for pollution indicators of waste waters and special waters (in Slovak). https://www.slov-lex.sk/pravne-predpisy/SK/ZZ/2010/269/ (cit. 08 June 2022)
Shaidan NH, Eldemerdash U, Awad S (2012) Removal of Ni(II) ions from aqueous solutions using fixed-bed ionexchange column technique. J Taiwan Inst Chem Eng 43:40–45. https://doi.org/10.1016/j.jtice.2011.06.006
Victor-Ortega MD, Ochando-Pulido JM, Martínez-Ferez A (2015) Impacts of integrated strong-acid cation exchange and weak-base anion exchange process for successful removal of saline toxicity from model olive mill wastewater. Ecol Eng 77:18–25. https://doi.org/10.1016/j.ecoleng.2015.01.005
Víctor-Ortega MD, Ochando-Pulido JM, Airado-Rodríguez D, Martínez-Ferez A (2016) Comparison between different ion exchange resins combinations for final treatment of olive mill effluent. Sep Purif Technol 158:374–382. https://doi.org/10.1016/j.seppur.2015.12.041
Wang H-G, Jia N, Liu W, Zhang M, Guo M (2016) Efficient and selective hydrothermal extraction of zinc from zinc-containing electric arc furnace dust using a novel bifunctional agent. Hydrometallurgy 166:107–111. https://doi.org/10.1016/j.hydromet.2016.10.013
Yu Z, Qi T, Qu J, Guo Y (2015) Application of mathematical models for ion-exchange removal of calcium ions from potassium chromate solutions by Amberlite IRC 748 resin in a continuous fixed bed column. Hydrometallurgy 158:165–171. https://doi.org/10.1016/j.hydromet.2015.10.015
Acknowledgements
The authors are grateful for the financial support provided through grant projects of Slovak Research and Development Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic APVV-14-0591 and Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic VEGA 1/0008/21.
Funding
This work was supported by grant projects of Slovak Research and Development Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic APVV-14-0591 and Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic VEGA 1/0008/21. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Remeteiová, D., Ružičková, S., Mičková, V. et al. Treatment of strong alkaline wastewater from neutral leaching of EAF dust by precipitation and ion exchange. Appl Water Sci 13, 138 (2023). https://doi.org/10.1007/s13201-023-01936-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01936-z