Abstract
Enormous quantities of organic wastes such as sewage sludge (SS) and aquatic weed compost (AWC) are produced in large quantities on the banks of Dal Lake Kashmir. It is a challenging task for authorities to manage them properly. Therefore, the study’s purpose was to evaluate these organic wastes agricultural use potential. The experiment was laid out in a randomized complete block design with three replications comprised of nine treatment combinations of SS, AWC and inorganic fertilizers. In the present study, the conjoint use of SS with chemical fertilizer recorded maximum build-up of soil microbial biomass carbon (MCB), urease and dehydrogenase activity with treatment T1. There were significant correlations between soil MCB and from urease and dehydrogenase activity (r2 = 0.95 and 0.97; P < 0.05), respectively. The micronutrient and heavy metal concentrations in kale exposed to SS and AWC were significantly higher than those in the untreated plants, with the highest concentration found in sole application of SS (T7). However, heavy metal concentrations were within the acceptable limits and did not overcome the maximum phytotoxic levels. The study’s finding leads to conclusion that SS along with chemical fertilizers (T1) can improve the enzymatic activity in soil, quality parameters and nutrient content in plants thereby enhancing the yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sewage sludge (bio-solids) generation in Kashmir is increasing at a faster rate and waste water treatment facilities with enhanced efficiencies are being developed. Similarly, macrophytes are being harvested manually and mechanically, providing a source for compost production (Nazir et al. 2021). These bio-solids and harvested aquatic weeds could easily be used for the restoration of soil fertility or for the production of quality vegetables, as they have good nutrient value (availability of NPK). The presence of heaps of sewage sludge (SS) and aquatic weed compost (AWC) on the banks of Dal Lake is a challenging task for Lake Management authorities for their proper disposal (Lone et al. 2013; Najar and Khan 2013). However, these organic wastes have a potential scope in agricultural practices by increasing the soil nutrient content; hence will increase the soil fertility. Thereby provides a solution to environmental nuisance and pollution (Goldan et al. 2022).
Conventional farm systems have been characterized by a high input of chemical fertilizers which leads to qualitative deterioration of soil and agricultural produce (Gitonga et al. 2021). However, growing environmental and ecological concerns, as well as understanding of the negative effects of inorganic fertilizers on crop yield, has generated more interest in using organic wastes for agricultural production (Zaffar et al. 2020). Further, broad application of agrochemicals interferes with the regular enzymatic activity of proliferating soil microorganisms and upsets the delicate equilibrium of the soil and ecological balance over the long term (Sawicka et al. 2020). There is need to shift to integrated, sustainable farming methods that utilize local, organic resources as fertilizers (Chukwuka and Omotayo 2009; Selim 2020).
Aquatic weed compost and sewage sludge are inevitable by-product of water bodies and wastewater treatment plants, respectively. These organic amendments generally contain beneficial compounds of potential environmental value such as nitrogen, phosphorus, and other plant nutrients like Mn, Cu, Mo and Zn depending on the specific nature of the sludge material (Arlo et al. 2021). The composting and use of these by-products has a positive impact on soil biological, chemical and physical qualities, and is being increasingly recognised as an environmentally appropriate disposal option (Dar et al. 2019).
Solid waste compost could be used as a soil conditioner without any phytotoxic effects on agricultural crops and abnormal increase in the level of Cu and Zn (Rasool et al. 2022). It has been suggested that enzyme activity, which are thought to be sensitive to heavy metals (Pb and Cd), could serve as possible markers for gauging the level of pollution in contaminated soil. (Lee et al. 2007, 2020). Micronutrients like Fe, Cu, Mn and Zn functions as precursors of many enzyme systems in plants. All of these are supplied by sewage sludge in adequate amount at proper time (Morkunas et al. 2018). Enzymatic activity is inhibited or rendered inactive when certain heavy metals interact with the sulphydral groups of enzymes. (Oves et al. 2016). However, organic matter and soil solutions contain organic matter-heavy metal fractions that are easily assimilated by plants. This would prevent the heavy metal from interacting directly with the active sites of enzyme, thus affecting the enzyme activity (Bartkowiak et al. 2020; Deforest et al. 2012).
Therefore, keeping in view the disposal challenges of sewage sludge and aquatic weed the present study was conducted to investigate the comparative effect of different combinations of sewage sludge and aquatic weed compost on soil enzymatic activity, nutrient and heavy metal status of kale (Brassica oleracea L.).
Materials and methods
Experiment design and agronomic practices
The present investigation was conducted at the Experimental Farm located in Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar campus for two consecutive years (Rabi 2016 and Rabi 2017). The experiment comprised of nine treatment combinations of sewage sludge, aquatic weed compost and inorganic fertilizers with triplicates in Randomized Complete Block Design (Table 1). The G.M. Dari variety (Brassica oleracea L. var. acephala) commonly known as kale was grown in the experimental field (Picture 1). The variety was selected keeping in view its growing capacity and market value. The field was levelled with uniform topography and suitable drainage mechanism. Vigorous and healthy seedlings of uniform size of kale were selected and transplanted in well prepared and fertilized plots at spacing of 30 × 15 cm. Climatically the experimental site is in mid to high altitude temperate zone characterized by hot summers and very cold winters. The mean weekly meteorological data collected from Meteorological observatory, Division of Agronomy SKUAST. The data revealed that average maximum and minimum temperatures during cropping seasons were 16.71, 2.70 °C (2016) and 15.94, 2.08 °C (2017), respectively. Total annual precipitation (rainfall) amounted to 383.70 and 428.10 mm during 2016 and 2017, respectively (Fig. 1).
Application of organic manures and inorganic fertilizers
Sewage sludge and aquatic weed compost were air dried and then mixed with soil in different proportion (w/w %) as specified in different treatments fifteen days before transplantation. The application of recommended doses of fertilizers (RDF: 90 kg N, 60 kg P2O5 and 60 kg K2O ha−1) for kale were given as basal application at the time of sowing.
Analytical details
Dried sewage sludge, aquatic weed compost and composite soil (collected from each plot at the depth of 1–15 cm before and after the crop harvest) samples were analysed for pH (Jackson 1973), soil texture (Piper 1966) and organic carbon (Walkley and Black 1934). Micro nutrients (Cu, Zn, Fe and Mn) and heavy metals (Cd and Pb) were analyzed by atomic absorption spectrophotometer (Jackson 1973) using di-acid digestion mixture (HNO3:HClO4, 4:1).
Ascorbic acid was determined using 2, 6 dichlorophenol indophenol dye. Dye factor was calculated by titrating 5 ml standard ascorbic acid plus 5 ml (3%) metaphosphoric acid against 2, 6 dichlorophenol till pink colour appeared and volume used was noted (Rangana 1986).
Ascorbic acid was estimated by taking 10 ml of sample, volume made upto 100 ml with 3% metaphosphoric acid and filtered. The aliquot of 10 ml was taken in a titration flask and titrated against 2, 6 di-chlorophenol indophenol till pink colour appeared (which persists for 15 s). Ascorbic acid was calculated using the following formula,
Total nitrogen content of the samples was estimated by modified Kjeldahl’s method (Jackson 1973). The sample was digested with sulphuric-salicylic acid. Organic and nitrate nitrogen is converted to ammonium sulphate and the ammonia gas is distilled into boric acid and titrated with standard sulphuric acid.
The total nitrogen was calculated by the following formula:
Total phosphorus content was estimated by vanado-molybdate-phosphoric method after digestion in diacid mixture (HNO3:HClO4 in the ratio of 9:4). During digestion phosphorus is converted to orthophosphates. These orthophosphates reacts with molybdate and vanadate and give yellow coloured unreduced vanado-molybdo-phosphoric complex in acid solution (Jackson 1973). The absorbance of solution was read after 30 min at 420 nm on spectrophotometer on blue filter.
The absorbance was then plotted against concentration and the total phosphorus was calculated from the following formula:
where C = Concentration obtained from standard curve (ppm).
Total potassium content in selected plant was estimated with the help of Flame photometer (Toth et al. 1948). The samples were digested in di-acid (HNO3:HClO4; 2:1). A series of standard solution of potassium (0.0, 5.0, 10.0 mg kg−1) were prepared. The flame photometer was calibrated with solution of highest concentration (i.e. 10.0 mg kg−1). Readings of other standards were then taken and curve was plotted. The samples were then read in flame photometer at 548 nm wavelength using potassium filter. The potassium content was calculated from the following formula:
where weight of plant sample = 0.5 g, total volume of plant digest = 50 ml, reading on flame photometer = R and potassium (ppm) as read from standard curve = U.
For estimation of soil urease activity 5 g of fresh soil sample was amended with 5 ml of urea solution and later incubated at 37 °C for 5 h. 50 ml of 2 M KCL-PMA solution and 30 ml of the colouring reagent were added. Total volume of sample was made 50 ml by adding water and later absorbance of red colour extract was determined by spectrophotometer at 527 nm. The amount of urease was computed from a calibration curve drawn under identical conditions (Bremner and Douglas 1971).
Soil dehydrogenase activity was determined by Tetrazolium salt method given by Klein et al. (1971). In this method 1 g of air dried soil was put in an air tight screw capped test tube. 0.2 ml of triphenyl tetrazolium chloride and 0.5 ml of glucose solution were added in the test tube. The tubes were sealed and incubated for 24 h at 28 °C in the dark and product triphenyl formazan (red colour) compound formed from the reduction of triphenyl tetrazolium chloride was extracted using methanol. Total volume of sample was made 10 ml by adding methanol. The triphenyl formazan concentration was determined by the spectrophotometric method at 485 nm.
The soil microbial biomass carbon (MCB) was determined by chloroform fumigation extraction method (Voroney et al. 1993). In the fumigation-extraction method, a direct measurement of carbon and other nutrients contained therein microbial biomass was carried out. Overnight fumigation with chloroform was carried out to kill all the organisms in soil samples. The microbial biomass constituents released by chloroform fumigation treatments can be extracted directly through chemical extract. MCB was measured in 10 ml of aliquots of K2SO4 extracts after oxidation with mixture of 2 ml of 0.2 N K2Cr2O7, 10 ml of H2SO4 and 5 ml of ortho-phosphoric acid at 100 °C for 30 min. and back titrated with ferrous ammonium sulphate.
where ECf is extractable carbon in the fumigated and non-fumigated (ECuf) soil sample and Kec = 0.35 represents the efficiency of extraction of organic carbon.
Plant micronutrients and heavy metals
The plant samples were digested in a di-acid mixture consisting of HNO3 and HClO4 to the known amount of plant material (1 g) 5 ml of conc. HNO3 was added and kept overnight. Next day 12 ml of di-acid mixture (HNO3:HClO4, 3:1) was added and digested on hot plate. The digestion process begins with the evolution of reddish brown fumes (NO2 gas) and the plant samples slowly start to dissolve and digested in a di-acid mixture. After few hours the plant samples dissolved completely in the digestion mixture and the solution was then evaporated until only 2 ml was left in the flask (Jackson 1973). The remaining digested material was diluted to 25 ml with distilled water and was then analyzed for the presence of heavy metals zinc, copper, manganese, iron, lead and cadmium by using atomic absorption spectrophotometer.
Statistical analysis
Data recorded during the experiment was subjected to statistical analysis using SPSS Version 18. The experimental results were subjected to ANOVA. The mean ± SE (standard error) were subjected to P < 0.05; Tukey’s test at ⍺ = 0.05.
Results and discussion
Nutrient characterization of of organic manures and non-amended soil
Table 2 shows the nutrient characterizations of SS, AWC and non-amended soil before crop transplantation. The SS and AWC used in this study were both acidic in nature and rich in SS and AWC. The concentrations of micronutrients and heavy metals in both SS and AWC were in the following order: Fe > Mn > Zn > Cu > Pb > Cd, whereas non-amended soil has the opposite trend: Fe > Mn > Cu > Zn > Pb, with cadmium concentration below detection level (BDL). Raw sewage sludge composition varies considerably and is primarily determined by the applied process technology, as well as the quantity and origin of raw wastewater. There is a high content of organic matter (up to 70%) in dehydrated sewage sludge, and its suitability for agricultural use is primarily determined by this content (Buta et al. 2021; Cheng et al. 2014; WWAP 2017), increasing soil fertility and promoting the development of plants and soil microbes (Samara et al. 2017; Tyrrell et al. 2019). The literature suggests that, in addition to the above advantages, sewage sludge also contains hazardous or potentially toxic heavy metals that impede its use in agriculture, as well as phyto-toxicity, soil pollution, and increase levels of toxic elements in food (Hussian et al. 2019; Iglesias et al. 2018).
During the study, we found micronutrients and heavy metal concentrations in sewage sludge used as organic amendments were very low, and far below the permissible levels. The non-amended soil was silty clay loam in texture and pH was neutral. Soil organic carbon and other nutrient concentrations were less in non-amended soils (Table 2). The results are in confirmatory with the finding of Qurashi (2012) who reported that nutrient concentration of initial soil before crop transplantation was very low and below the permissible limit. The experimental results as mentioned above indicated that soil before crop transplantation needs additional fertilizers for proper growth and development of crop.
Soil enzymatic activity and microbial biomass carbon
A critical perusal of the data presented in Fig. 2a regarding the soil MCB, it is evident that using SS combined with chemical fertilizer, resulted in the maximum buildup of soil MCB (µg/g) with treatment T1 (421.06 ± 2.25 and 427.82 ± 1.24) followed by T2 as compared to T8. Increased microbial biomass carbon content recorded in the conjoint use of SS with chemical fertilizers could be attributed to the supply of additional mineralizable substratum and readily hydrolysable carbon for microbial growth and activity (Kunda et al. 2016; Dhaliwal et al. 2022). Soil microbial population behavior depends on the quality and quantity of residues added. Fernando et al. (2005) demonstrated that the highest rates of sewage sludge application resulted in a 220% increase in atmospheric CO2 fluxes when compared to the controls.
We observed significant differences in urease and dehydrogenase activity in soil when SS, AWC, and inorganic fertilizers were applied. Moreover, all the combinations were statistically at par with each other treatments maximum urease (μg urea hydrolysed/g soil/2 h) and dehydrogenase (µg TPF/g/24 h) activity were recorded in T1 (65.76 ± 0.25 and 67.80 ± 0.15) and (48.26 ± 1.15 and 49.66 ± 0.28) followed by treatment T2 as compared to RDF (T8), respectively (Fig. 2b, c). While as, treatment T9 recorded lowest urease and dehydrogenase activities during 2016 and 2017. The increase in enzymatic activities may be attributed to the reason that addition of balanced application of organic and chemical fertilizers causes an increase in substrate thereby increases the diversity and activity of microorganisms accompanied by better urease and dehydrogenase activity (Lazcano et al. 2013).
The relation between soil MCB and urease activity yielded the linear regression equation: y = 0.0993x + 21.332 (r2 = 0.9088; P < 0.05), where x = soil MCB and y = urease activity. The linear regression equation between soil MCB and dehydrogenase activity was: y = 0.107x + 0.2988 r2 = 0.9468; P < 0.05), x = soil MCB and y = dehydrogenase activity Fig. 3. The urease and dehydrogenase activity values followed the same trend as the values obtained for soil MCB. There were significant correlations between soil MCB and from urease and dehydrogenase activity (r2 = 0.95 and 0.97; P < 0.05), respectively. As per previous findings, the enzyme activity was greater in forest soils compared to cultivated soils, and the availability of fresh soil organic matter (SOM) enhanced the microbial activity in forest soil and increased enzyme activity (De Medeiros et al. 2015; Meena and Rao 2021; Vinhal-Freitas et al. 2017).
Quality analysis of kale
Ascorbic acid is a major antioxidant agent in plants. It is involved in many enzymatic activities like hydroxylation of proline to hydroxyproline, cyclic oxidation reduction reactions and protects the plants against the harmful side-effects of heavy metals (Agar et al. 2020). The pooled data presented in Fig. 4a indicated that sole application of SS and AWC has a significant effect on ascorbic acid content. Among all treatments, maximum ascorbic acid content was recorded in treatment T7 (122.47 ± 1.38) as compared to sole application of RDF (T8; 106.18 ± 0.68). Lowest ascorbic acid content was recorded when no fertilizer amendments were carried out (T9; 71.15 ± 0.27). This may be due to the fact that ascorbic acid, a natural antioxidant, may have scavenged the effect of free radicals generated by heavy metals (Alarmi et al. 2018). Furthermore, hydrophilic antioxidants, such as ascorbic acid (AsA), play an important role in scavenging reactive oxygen species (Sofy et al. 2020). According to our findings, Pb-stressed kale leaves contained significantly higher levels of ascorbic acid (AsA) than non-Pb-stressed leaves. Our results are in conformity with the finding of Akladious and Mohamed (2018).
The pooled data presented in Fig. 4b, c, d reveal significant variation with respect to total nitrogen (N), phosphorus (P) and potassium (K) content in kale crop. In treatment T1, the highest concentrations of NPK were found (1.51 ± 0.01, 0.43 ± 0.01 and 1.15 ± 0.001) followed by treatment T2 compared to T8, respectively. The elevated levels of total NPK due to conjunctive use of organic manure with chemical fertilizers can be attributed to good carbon/nitrogen and carbon/phosphorus ratios, organic matter buildup, and leaching losses. Improved soil fertility might increase the ability of soil to absorb more nutrients.
Moreover, synergistic and stimulatory effects of organic amendments may result in a better root formation and enhanced photosynthetic activity of plants (Kariithi 2018; Sebnie et al. 2018). Wierzbowska et al. (2016) observed that elevated nitrogen and phosphorus levels encourage potassium uptake, and that mineralization and solubilization possibly facilitated their uptake. It could be related to the supply of nitrogen, phosphorus, and potassium, since the chemical fertilizers present a continuous supply of nutrients in the early stages of the crop and the slow and incessant release of nutrients from organic sources in the later stages of the crop. Our finding was also supported by Verma et al. (2021) who stated that application of sewage sludge along with recommended doses of NPK significantly enhanced the uptake of nitrogen, phosphorus, and potassium in wheat (Triticum aestivum L.) as compared to sewage sludge alone.
Micro-nutrients and heavy metals in kale
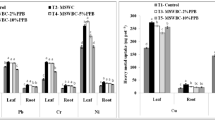
The data presented in Fig. 5 revealed that application of higher levels of SS and AWC recorded significantly elevated levels of Zn, Mn, Cu, Fe, Pb and Cd in kale. While comparing nine treatments significantly maximum Zn, Mn, Cu, Fe, Pb and Cd (ppm) concentrations were reported in 100% SS (26.94 ± 0.46 and 28.49 ± 0.16), (31.21 ± 0.48 and 32.50 ± 0.20), (8.75 ± 0.29 and 9.07 ± 0.06), (112.07 ± 0.50 and 117.78 ± 0.74), (2.42 ± 0.05 and 2.53 ± 0.03) and (0.063 ± 0.002, and 0.067 ± 0.006) as compared to un-amended soil, respectively, during two successive years. The concentration of these metals in kale treated with different rates of SS and AWC were ranked in the following order: Fe > Mn > Zn > Cu > Pb > Cd. This was perhaps due to the fact that these metals in soil are present in different forms such as solid phases, free ions, and soluble organic mineral complexes. Hence, SS and AWC addition to soils could affect the availability of these metals as initial concentration of these metals was found high in both organic fertilizers (Belhaj et al. 2016; Rastetter and Gerhardt 2017). Our results are also in conformity with the finding of Eid et al. (2018) who stated that elevated levels of SS modified the chemical properties of soil, thus increased the bioavailability of heavy metals in soil and subsequently a significant increase in most heavy metal in different tissues of wheat was observed.
Generally, in this study, the distribution of heavy metals in plant bound to the mobile fractions increased gradually over successive rates of sewage sludge (Fig. 5). Correlation coefficients (r) shown in Fig. 6 indicate high positive correlation between the metals and sewage sludge dosage, i.e., by increasing the proportion of sewage sludge from 20 to 40 to 100% with respect to RDF an increase in accumulation of metals were reported. Eid et al. (2017) reported that the application of sewage sludge to soil at a rate of 40 g kg−1 or less may be beneficial for spinach, soil health and avoid the risk to human health. At elevated sewage sludge rates (e.g., 50 g kg−1) higher accumulation of heavy metals in crop is a serious concern for human health. Chopra et al. (2017) also reported that elevated levels of heavy metals, i.e., Cd (0.35 and 0.32 mg kg−1), Cu (1.28 and 1.26 mg kg−1), Fe (2.25 and 2.20 mg kg−1), Mn (0.78 and 0.74 mg kg−1) and Zn (2.14 and 2.05 mg kg−1) in tomato (Lycopersicon esculentum L.) were recorded in sole application of sewage sludge (T6). In the current study, concentrations of most of the heavy metal were below or within the permissible limits and did not exceed the maximum levels of phytotoxic at any of the SS amendment rates (Table 3). Kabata-Pendias (2011) reported the concentrations of all of the monitored heavy metals in C. olitorius shoots (except Fe) did not reach the critical levels.
Conclusion
It is concluded that, among nine treatments under study T1 (20% SS + 80% RDF) and T2 (40% SS + 60% RDF) proved superior over rest of the treatments with respect to improvement in quality attributes, macro-nutrient uptake by plant and enzymatic activity of soil. The integrated use of inorganic fertilizers with SS at all levels was found to be better than integrated use of inorganic fertilizers with AWC in enhancing the yield and nutrient availability. Sole application of SS and AWC as fertilizers may have hazardous impact on soil/plant in terms of bioaccumulation of heavy metals as lead content in plant exceeds the critical toxicity level. In order to avoid their accumulation in the food chain, it is, however, recommended that agricultural products be routinely tested and processed for heavy metal levels. Utility of SS and AWC as organic manure will reduce the hazardous environmental impact caused due to their disposal.
References
Agar G, Taspinar MS, Yildirim E, Aydin M, Yuce M (2020) Effects of ascorbic acid and copper treatments on metallothionein gene expression and antioxidant enzyme activities in Helianthus annuus L. exposed to chromium stress. J. Plant Growth Regul 39(2):897–904. https://doi.org/10.1007/s00344-019-10031-0
Akladious SA, Mohamed HI (2018) Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci Hortic 236:244–250. https://doi.org/10.1016/j.scienta.2018.03.047
Alarmi SA, Siddiqui MH, Al-Khaishany MYY, Khan MN, Ali HM, Alaraidh IA, Alsahli AA, Al-Rabiah H, Mateen M (2018) Ascorbic acid improves the tolerance of wheat plants to lead toxicity. J Plant Interact 13(1):409–419. https://doi.org/10.1080/17429145.2018.1491067
Arlo L, Beretta A, Szogi AA, Del Pino A (2021) Biomass production, metal and nutrient content in sorghum plants grown on soils amended with sewage sludge. Heliyon 8(1):e08658. https://doi.org/10.1016/j.heliyon.2021.e08658
Awashthi SK (2000) Prevention of food adulteration Act No. 37 of 1954 central and state rules as amended for 1999, Ashoka Law House, New Delhi, India
Bartkowiak A, Lemanowicz J, Lamparski R (2020) Assessment of selected heavy metals and enzyme activity in soils within the zone of influence of various tree species. Sci Rep 10:14077. https://doi.org/10.1038/s41598-020-69545-3
Belhaj D, Elloumi N, Jerbi B, Zouari M, Abdallah FB, Ayadi H, Kallel M (2016) Effects of sewage sludge fertilizer on heavy metal accumulation and consequent responses of sunflower (Helianthus annuus). Environ Sci Pollut Res 23(20):20168–20177. https://doi.org/10.1007/s11356-016-7193-0
Bremner JM, Douglas LA (1971) Inhibition of urease activity in soils. Soil Bio Biochem 3:297–307. https://doi.org/10.1016/0038-0717(71)90039-3
Buta M, Hubeny J, Zieliński W, Harnisz M, Korzeniewska E (2021) Sewage sludge in agriculture–the effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—a review. Ecotoxicol Environ Saf 214:112070. https://doi.org/10.1016/j.ecoenv.2021.112070
Cheng M, Wu L, Huang Y, Luo Y, Christie P (2014) Total concentrations of heavy metals and occurrence of antibiotics in sewage sludges from cities throughout China. J Soils Sediment 14:1123–1135. https://doi.org/10.1007/s11368-014-0850-3
Chopra AK, Payum T, Srivastava S, Kumar V (2017) Effects of integrated nutrient management on agronomical attributes of tomato (Lycopersicon esculentum L.) under field conditions. Arch Agric Environ Sci 2(2):86–91
Chukwuka KS, Omotayo OE (2009) Soil fertility restoration potentials of Tithonia green manure and water hyacinth compost on a nutrient depleted soil in South Western Nigeria using Zea mays L. as test crop. Res J Soil Bio 1(1):20–30
CPCB (2005) Parivesh sewage pollution-news letter, Central pollution control board, Ministry of Environment and Forests, Govt. of India, East Arjun Nagar, Delhi, 110032
Dar ZA, Bhat JIA, Lone FA, Ali T, Mir SA, Khan SH, Singh P (2019) Impact of sewage sludge and Dal weed compost on yield and quality attributes of kale (Brassica oleracea var. acephala L.) in Kashmir. J Pharmacogn Phytochem 8(1):853–856
De Medeiros EV, Notaro KA, De Barros JA, Moraes WS, Silva AO, Moreira KA (2015) Absolute and specific enzymatic activities of sandy entisol from tropical dry forest, monoculture and intercropping areas. Soil Tillage Res 145:208–215
DeForest JL, Smemo KA, Burke DJ, Elliott HL, Becker JC (2012) Soil microbial responses to elevated phosphorus and pH in acidic temperate deciduous forests. Biogeochemtry 109:189–202
Dhaliwal SS, Gupta R, Singh AK, Naresh RK, Mandal A, Singh UP, Mahajan NC (2022) Impact of cropping systems on pedogenic distribution and transformations of micronutrients, plant accumulation and microbial community composition in soils: a review. Trop Ecol. https://doi.org/10.1007/s42965-022-00272-8
Eid EM, El-Bebany AF, Alrumman SA, Hesham A, Taher MA, Fawy KF (2017) Effects of different sewage sludge applications on heavy metal accumulation, growth and yield of spinach (Spinacia oleracea L.). Int J Phytorem 19:340–347
Eid EM, Alrumman SA, El-Bebany AF, Fawy KF, Taher MA, Hesham A, El-shaboury GA, Ahmed MT (2018) The evaluation of sewage sludge application as a fertilizer for broad bean (Faba sativa Bernh.) crops. Food Energy Secur 7(3):1–13
FAO/WHO (2007) Summary of evaluations performed by the joint FAO/WHO expert committee on food additives (JECFA 1956-2007) (first through 68th meetings), Food and Agriculture Organization of the United Nations and the World Health Organization, ILSI Press International Life Sciences Institute, Washington, DC., USA
Fernandes SAP, Bettiol W, Cerri CC, Camargo P (2005) Sewage sludge effects on gas fluxes at the soil–atmosphere interface, on soil d13C and on total soil carbon and nitrogen. Geoderma 25:49–57
Gitonga NM, Njeru EM, Cheruiyot R, Maingi JM (2021) Genetic and morphological diversity of indigenous bradyrhizobium nodulating soybean in organic and conventional family farming systems. Front Sustain Food Syst 4:606618. https://doi.org/10.3389/fsufs.2020.606618
Goldan E, Nedeff V, Barsan N, Culea M, Tomozei C, Panainte-Lehadus M, Mosnegutu E (2022) Evaluation of the use of sewage sludge biochar as a soil amendment a review. Sustainability 14:5309. https://doi.org/10.3390/su14095309
Hussain A, Priyadarshi M, Dubey S (2019) Experimental study on accumulation of heavy metals in vegetables irrigated with treated wastewater. Appl Water Sci 9:122. https://doi.org/10.1007/s13201-019-0999-4
Iglesias M, Marguí E, Camps F, Hidalgo M (2018) Extractability and crop transfer of potentially toxic elements from mediterranean agricultural soils following long-term sewage sludge applications as a fertilizer replacement to barley and maize crops. Waste Manag 75:312–318. https://doi.org/10.1016/j.wasman.2018.01.024
Jackson ML (1973) Soil chemical analysis. Prentice-Hall, New Delhi
Kabata-Pendias A (2011) Trace elements in soils and plants. CRC Press, Boca Raton, FL
Kariithi T (2018) Effect of organic and inorganic fertilizers on growth, yield and quality of Amaranths in Kiambu County, Kenya, Ph.D thesis submitted to school of agriculture and enterprise development in Kenyatta University, Kenya
Klein DA, Loh TC, Goulding R (1971) A rapid procedure to evaluate dehydrogenase activity of soils low in organic matter. Soil Biol Biochem 3:385–387
Kundu DK, Mazumdar SP, Ghosh V, Saha AR, Majumdar B, Ghorai AK, Behera MS (2016) Long-term effects of fertilizer and manure application on soil quality and sustainability of jute-rice-wheat production system in Indo-Gangetic plain. J Appl Nat Sci 8(4):1793–1800
Lazcano C, Gómez-Brandón M, Revilla P, Domínguez J (2013) Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol Fertil Soils 49(6):723–733. https://doi.org/10.1007/s00374-012-0761-7
Lee SH, Lee S, Kim DY, Kim JG (2007) Degradation characteristics of waste lubricants under different nutrient conditions. J Hazard Mater 143:65–72
Lee SH, Kim MS, Kim JG, Kim SO (2020) Use of soil enzymes as indicators for contaminated soil monitoring and sustainable management. Sustainability 12:8209. https://doi.org/10.3390/su12198209
Lone FA, Sabia Z, Nousheen Q, Rather AQ, Kirmani NA (2013) Studies on efficacy of sewage sludge as an agricultural supplement for the assessment of growth performance of Brinjal (Solanum melongena var. Local long). Nat Environ Pollut Technol 12(2):367–370
Meena A, Rao KS (2021) Assessment of soil microbial and enzyme activity in the rhizosphere zone under different land use/cover of a semiarid region India. Ecol Process 10:16. https://doi.org/10.1186/s13717-021-00288-3
Morkunas I, Woźniak A, Mai VC, Rucińska-Sobkowiak R, Jeandet R (2018) The role of heavy metals in plant response to biotic stress. Molecules 23(9):2320
Najar IA, Khan AB (2013) Management of fresh water weeds (macrophytes) by vermicomposting using Eisenia fetida. Environ Sci Pollut Res 20:6406–6417. https://doi.org/10.1007/s11356-013-1687-9
Nazir A, Bhat JIA, Dar ZA, AlTawaha ARM (2021) Influence of different sources of organic and inorganic fertilizers on tomato (Solanum lycopersicum mill) growth, quality and soil properties. Adv Environ Biol 15(1):8–15. https://doi.org/10.22587/aeb.2021.15.1.2
Oves M, Saghir KM, Huda QA, Nadeen FM, Almeelbi T (2016) Heavy metals: biological importance and detoxification strategies. J Bioremediat Biodegrada 7(2):334
Piper CS (1966) Soil and plant analysis. Hans Publisher, Bombay
Qurashi F (2012) Response of Kale (Brassica oleracea var. acephala) to different levels of farm yard manure and inorganic nitrogen on yield, quality and nitrate accumulation under Eutrochrepts, Ph.D thesis submitted to Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar India
Rangana S (1986) Manual for analysis of fruit and vegetable products. McGraw Hill, New Delhi, pp 94–98
Rasool A, Kanagaraj T, Mir MI, Zulfajri M, Ponnusamy VK, Mahboob M (2022) Green coalescence of CuO nanospheres for efficient anti-microbial and anti-cancer conceivable activity. Biochem Eng J 187:108464. https://doi.org/10.1016/j.bej.2022.108464
Rastetter N, Gerhardt A (2017) Toxic potential of different types of sewage sludge as fertilizer in agriculture, Ecotoxicological effects on aquatic, sediment and soil indicator species. J Soils Sediments 17:106–121
Samara E, Matsi T, Balidakis A (2017) Soil application of sewage sludge stabilized with steelmaking slag and its effect on soil properties and wheat growth. Waste Manag 68:378–387. https://doi.org/10.1016/j.wasman.2017.06.016
Sawicka B, Krochmal-Marczak B, Pszczółkowski P, Bielinska EJ, Wójcikowska-Kapusta A, Barbas P, Skiba D (2020) Effect of differentiated nitrogen fertilization on the enzymatic activity of the soil for sweet potato (Ipomoea batatas L. [Lam.]) cultivation. Agronomy 10:1970
Sebnie W, Mengesha M, Girmay G, Feyisa T (2018) Response of garlic (Allium sativum L.) to nitrogen and phosphorus under irrigation in Lasta district of Amhara Region, Ethiopia. Cogent Food Agric 4(1):1532862
Seli MM (2020) Introduction to the integrated nutrient management strategies and their contribution to yield and soil properties. Int J Agron. https://doi.org/10.1155/2020/2821678
Sofy MR, Seleiman MF, Alhammad BA, Alharbi BM, Mohamed HI (2020) Minimizing adverse effects of pb on mazie plants by combined treatment with Jasmonic, salicylic acids and proline. Agronomy 10:699. https://doi.org/10.3390/agronomy10050699
Toth SJ, Prince AI, Wallace A, Mikkelsen DS (1948) Rapid quantitative determination of eight mineral elements in plant tissues by a systematic procedure involving use of flame photometer. Soil Sci 66:459–466
Tyrrell C, Burgess CM, Brennan FP, Walsh F (2019) Antibiotic resistance in grass and soil. Biochem Soc Trans 47:477–486. https://doi.org/10.1042/BST20180552
USEPA (2010) Risk-based concentration table. United States Environmental Protection Agency, Washington DC
Verma AK, Mahesh C, Meena SP, Datta BS, Dwivedi D, Golui VK, Singh SM, Kumar A, Louhar G, Meena R, Tigga P (2021) Effect of long-term integration of sewage-sludge and fertilizers on wheat productivity, profitability and soil fertility. J Indian Soc Soil Sci 69(1):80–85
Vinhal-Freitas IC, Correa GF, Wendling B, Bobulska L, Ferreira AS (2017) Soil textural class plays a major role in evaluating the effects of land use on soil quality indicators. Ecol Indic 74:182–190. https://doi.org/10.1016/j.ecolind.2016.11.020
Voroney RP, Winter JP, Beyart RP (1993) Soil microbial biomass C and N. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis, Chelsea, pp 277–286
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wierzbowska J, Sienkiewicz S, Krzebietke S, Sternik P (2016) Sewage sludge as source of nitrogen and phosphorus for Virginia fanpetals. Bulg J Agr Sci 22(5):722–727
WWAP (2017) The United Nations world water development report 2017, Wastewater: the untapped resource. Cambridge University Press, Paris
Zaffar S, Farooq S, Qazi HA, Jaweed TA, Kadam AK, Lone FA (2020) Evaluation of nutrient status of kale and spinach as affected by sewage sludge and mineral fertilizers. J Plant Nutr 43(17):2633–2644
Acknowledgements
Authors are grateful to the staff members of Division of Environmental Sciences, SKUAST-K, for extending their help to use the department laboratory for computational purpose. The authors would also like to thank Directorate Research, SKUAST-K for providing agricultural land for carrying out experimental trial.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Authors equally contributed to this research paper.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dar, Z.A., Bhat, J.I.A., Qazi, G. et al. Municipal sewage sludge, aquatic weed compost on soil enzymatic activity and heavy metal accumulation in Kale (Brassica oleracea L.). Appl Water Sci 13, 60 (2023). https://doi.org/10.1007/s13201-022-01855-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01855-5