Abstract
The current work investigated the efficacy of the hybrid treatment process of electrocoagulation and adsorption in removing hydroxyzine (HDZ) from polluted aqueous solutions. Response surface methodology (RSM) was used to optimize the operating parameters based on the sub-category of central composite design (CCD). The significance of variables, interactions, and quadratic effects was investigated through analysis of variance (ANOVA). The value of determination coefficient (R2), Adjusted R2 (Adj.R2) and predicted R2 (Pred.R2) were 0.9855, 0.9791, and 0.9743, respectively; also, p-value of P < 0.0001, and F-value of 65.91 were obtained. The obtained results revealed that the removal efficiency of 99.3% and electrical energy consumption of 0.438 kWh m−3 were achieved at the optimum treatment condition of initial HDZ concentration of 25.0 mg L−1, pH solution of 8.0, the current density of 12.0 mA cm−2, reaction time of 15.0 min, and chitosan dosage of 0.03 g L−1. According to the Pareto analysis, the initial HDZ concentration, solution pH, current density, and reaction time’s contribution to the HDZ removal were 22.61%, 38.99%, 19.36%, and 9.43, respectively. Furthermore, the contributions of solution pH and reaction time with the quadratic effects were 3.43% and 6.19%, respectively. Thus, the pH solution revealed the highest contribution to the removal process. Overall, HDZ removal by the hybrid treatment process of EC and AD revealed a good efficiency; also, it can be potentially presented as a promising process for treating polluted water.
Similar content being viewed by others
Introduction
In recent years, the improvement of technology and analytical methods has led to detecting a new group of pollutants in the water, wastewater, soil, and air, called emerging pollutants (EPs). EPs have caused great concern in human societies due to the adverse effects on human health and destructive environmental effects (Khan et al. 2020). EPs include human and veterinary pharmaceutical, cosmetic products, plasticizers, surfactants, and herbicides whose toxicological effects are relatively unidentified. In the water and soil ecosystems, traces of pharmaceuticals compounds and their metabolites have been detected even in low concentrations (Stavbar et al. 2017). H1 antihistamines are a group of widely used drugs prescribed for treating and preventing allergy-based diseases. Hydroxyzine (HDZ) is one of the first suggested antihistamines for controlling, managing, and preventing skin irritation, urticaria, atopic dermatitis, and dermatosis. Moreover, HDZ is one of the most effective antihistamines in treating chronic itching, eczema, and skin allergies (Luján et al. 2020; Thomas et al. 2019).
Even in a low concentration, the damaging effects of pharmaceuticals in the environment were their toxic effects and endocrine disruption in humans. Moreover, bioaccumulation in the food chain, disruption in the photosynthetic function of aquatic plants, and disruption in the natural balance of ecosystems cause great concern to communities' public health. (Zhao et al. 2017, Iqbal 2016). Therefore, it is essential to remove these environmental pollutants using efficient treatment technologies. Several techniques were developed and applied to minimize their contents in waters and wastewater, such as Fenton, electrochemical degradation (Xia and Dai 2018, Abbas et al. 2018), ozonation (Kharel et al. 2020; El-Taliawy et al. 2018), membrane processes (Mamo et al. 2018; Rosman et al. 2018), and adsorption by application of activated carbons (Köpping et al. 2020; Jaria et al. 2020).
The electrocoagulation (EC) process is one of the most efficient water and wastewater purification methods due to its high efficiency, safe, green, and environment-friendly technique. This method released metal ions from the anode electrode, and H2O was hydrolyzed at the cathode electrode to generate H2 gas. Finally, the metal ions form flocculates that trap the pollutants while the H2 gas floats these particles (Tahreen et al. 2020, Vepsäläinen and Sillanpää 2020). So far, various pollutants from different wastewaters have been successfully treated using the electrocoagulation process, such as dye (Bilińska et al. 2020), heavy metal (Kim et al. 2020), pharmaceutical compounds (Lu et al. 2021), pesticide (Pinedo-Hernández et al. 2020), and other pollutants (Isik et al. 2020; Syam Babu et al. 2020).
Response surface methodology (RSM), based on the sub-category of central composite design (CCD), is a set of mathematical and statistical operations that can logically reduce the number of experiments. The developed model can simultaneously measure the main effects of variables and their interactions and quadratic effects (Yang et al. 2020). To the best of our knowledge, this is the first time chitosan has been employed as an efficient adsorbent besides the electrocoagulation process as a hybrid treatment technique for removing hydroxyzine using the CCD in response surface methodology (RSM). Considering the high removal efficiency, the developed treatment process could be a promising, simple, cost-effective procedure for removing HDZ from polluted aqueous solutions. The possible mechanism of the treatment process is explained to understand the exact procedure that occurred throughout the process. Eventually, the aims of the study are as follows: (1) to investigate the effects of various operating parameters such as initial HDZ concentration, solution pH, current density, reaction time, and chitosan dosage, (2) optimization of the HDZ removal efficiency and investigation of the main, interaction, and cubic effects of critical operating parameters, including initial HDZ concentration, chitosan dosage, contact time, current density, and solution pH, (3) study and comparison of electrocoagulation, adsorption, and hybrid treatment processes.
Materials and methods
Chemicals
Hydroxyzine hydrochloride (C21H27ClN2O2.2HCl, ˃98.5%) was obtained from Pursina pharmaceutical Co, Tehran, Iran. Other chemicals such as chitosan (2-Amino-2-deoxy-(1 → 4)-β-D-glucopyranan, high purity), hydrochloric acid fuming (HCl, 37.0%), sodium sulfate (Na2SO4, ≥ 99.0%), sodium hydroxide (NaOH, ≥ 98.0%), and sodium chloride (NaCl, ≥ 99.9%) were obtained from Merck Company.
Experiment setup and analytical methods
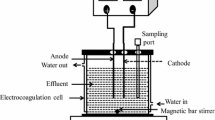
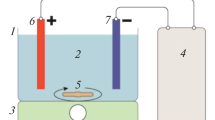
The HDZ removal process was done on a lab scale and at ambient temperature. The experimental set-up for electrocoagulation (EC) of the wastewater consisted of two electrodes, a glass reactor (with 9 cm height and 7 cm internal diameter), and a DC power supply. The iron electrodes with dimensions of 4.0 cm high, 2.0 cm wide, and 1.0 mm thick were prepared and used after polishing with sandpaper and washing with 20% hydrochloric acid. Before each experiment, 250 mL of the synthetic wastewater was transferred into the reactor. Two iron electrodes were placed in the electrochemical reactor in parallel and with a distance of 3 cm. 50 mM of sodium chloride (NaCl) as the supporting electrolyte was added to the reactor. Using the magnetic stirrer, the working solution was in the reactor at 150 rpm. The effects of all variables, including HDZ concentration, solution pH, current density, and reaction time, were studied at different conditions as listed in Table 1. At the end of the EC process, the samples were filtered using a 0.2 µm filter and investigated for remaining concentrations of HDZ using a spectrophotometer UV/Vis at 235 nm. The HDZ removal efficiency is defined as follows in Eq. 1 (uz Zaman et al. 2021):
where Ci and Ct (mg L–1) denote the concentration of HDZ before and after the treatment process at time t, respectively.
Electrical energy consumption (EEC) in the EC process was calculated as follows in Eq. 2 (Minas et al. 2017; Sharif et al. 2021):
where U, I, t, and V are applied voltage (U), applied electrical current (A), reaction time (h), and volume of sample (L), respectively.
Process variables and experimental design
Four variables, including initial HDZ concentration, solution pH, current density, and reaction time, were employed for making the central composite design (CCD). The relation between the mentioned variables was estimated as follows (Almomani and Bohsale 2020, Alibabaei et al. 2021):
where Y is the response; Xi and Xj are the variables; β0 is the constant coefficient; βi, βii, and βij are the interaction coefficients of linear, quadratic, and interaction effects, respectively; n is the number of studied factors, and ɛ is the error. The range and the levels of the variables investigated in this study are given in Table 1.
Results and discussion
RSM approach and statistical analysis
Response surface methodology (RSM) was used to evaluate the main effects of parameters, their simultaneous interactions, and quadratic effect to achieve the optimum condition for the treatment process. Experimental data, including the responses, are shown in Table 2.
The quadratic model Adjusted R2 (Adj.R2) and predicted R2 (Pred.R2) are 0.9791 and 0.9743, respectively. Accordingly, it was shown that the predicted model could describe nearly 97.9% of the total variation. The results from the analysis of variance (ANOVA) for the model are given in Table 3. The model F-value of 152.7 and the p-value < 0.0001 also recommend that the model was significant.
Experimental results were fitted to a polynomial model. The polynomial model in terms of coded factors only for the significant factors (p-value ≤ 0.05) is as below:
where X1, X2, X3, and X4 are initial HDZ concentration, solution pH, current density, and reaction time, respectively. The intercept parameter (β0 = 71.95%) denotes the average percentage of HDZ removal when all factors are fixed at their center point. However, two variables revealed quadratic effects (\({\mathrm{X}}_{2}^{2},\) and \({\mathrm{X}}_{4}^{2}\)) significantly influencing the response. These effects are further described in the next section. To determine the exact contribution of each operating parameter to the response, the graphical Pareto analysis was demonstrated according to Eq. 5:
where bi represents the estimation of the main effect of the variables. The main effects of X1, X2, X3, and X4 denote the initial HDZ concentration, solution pH, current density, and reaction time, respectively. As seen in Fig. 1, the contribution of the main variables to the HDZ removal was 22.61%, 38.99%, 19.36%, and 9.43, respectively. Furthermore, the contributions of the X2 and X4 with the quadratic effects of \({\text{X}}_{2}^{2} ,{ }\) and \({\text{ X}}_{4}^{2}\) to the HDZ removal efficiency were 3.43% and 6.19%, respectively. Thus, it could be inferred that the pH solution, initial HDZ concentration, current density, reaction time, the quadratic effect of reaction time, and pH solution were the most contributing factor to HDZ removal, respectively.
Data were analyzed to check the normality of the residuals, as shown in Fig. 2. It can be seen from Fig. 2-a that data on this plot were reasonably close to a straight line (R2 = 0.9855). The correlation between the actual and predicted values of response (% Y) for the removal of HDZ is shown in Fig. 2-b. Therefore, the obtained results propose that the model accurately predicted the removal efficiency of the treatment process throughout the experimental runs because residual results were distributed near the diagonal.
Figure 3 shows that the removal efficiency of HDZ decreases by 22.5% (83.2% to 60.7%) by increasing the initial HDZ concentration from 5 to 50 mg L−1 at the center point of the variables, including 12.5 mA cm−2 current density, solution pH of 7, and 16.5 min reaction time. According to the Pareto analysis (Fig. 1), the initial concentration of HDZ was identified as the second parameter affecting the removal efficiency of HDZ. This phenomenon is because of the number of coagulation agents (metal hydroxides) formed during the EC process that has a remarkable capacity to trap, sediment, and remove HDZ molecules. At higher concentrations of HDZ, because the formed metal hydroxides are constant, the removal efficiency of hydroxyzine was decreased.
Solution pH with a contribution of 38.9% showed the most significant effect on the removal efficiency of HDZ during the treatment process. As seen in Fig. 4, the removal efficiency of HDZ increased from 48.4% to 78.1 by increasing the solution pH from 4 to 10. At the center point of the operational parameters, including 27.5 mg L−1 initial HDZ concentration, 12.5 mA cm−2 current density, and 16.5 min reaction time, the maximum removal efficiency was obtained at pH 9.5 (85.1%). HDZ has two main pKa at 2.47 and 6.95. Due to the amine group's protonation, the HDZ molecules exist in a cationic form at a pH lower than 2.47. On the other hand, at the low pH condition, iron hydroxides, which are the hydrolysis products of Fe3+, are soluble; therefore, they cannot absorb the HDZ molecules. At pH between 2.47 and 6.95, HDZ exists as the zwitterion due to the loss of the proton from the phenolic moiety. Therefore, the removal efficiency is also increased in this range. The maximum removal occurred at a pH range of 7.0–10.0. At the pH range of 6.7–10.0, HDZ prevails as the anionic form due to the loss of the proton from the carbonyl system and phenolic moiety. However, Fe(OH)3 and [Fe(OH)2]+ are the main species of Fe3+ hydrolysis reactions (see reactions 6, 7, 8, 9, 10, 11, 12, 13). Therefore, the efficiency of removal in this range increases (Sandoval et al. 2021, Khandegar and Saroha 2013, Pulkka et al. 2014).
Additional mechanism suggested for the production of iron species occurs as follows:
Current density and reaction time are two basic parameters that affect the reaction rate and generation of coagulation agents during the treatment process. The variation in the value of HDZ removal efficiencies with changing the applied reaction time (3–30 min) at different current densities (5–20 mA cm−2) is shown in Fig. 5. As shown in Fig. 5, the removal efficiency of HDZ was increased by increasing the current density. The obtained results revealed that 61.5% of the HDZ could be removed at the low current density of 5 mA cm−2, where increasing the current density up to 20 mA cm−2 increased the removal efficiency of HDZ to the value of 82.3% at the center point of the operational parameters, including the solution pH of 7, 27.5 mg L−1 initial HDZ concentration and 16.5 min reaction time. Increasing current density increased the generation rate of coagulant agents on the anode during the EC process.
Due to the increased generation of coagulation agents rate, the removal efficiency of HDZ is improved. In other words, the higher current density will generate a higher number of flocs (coagulant agents), which will trap and sediment the HDZ molecules and enhance the HDZ removal efficiency. In the investigation of the treatment of oil tanning effluent by electrocoagulation by Lakshmi et al., with increasing current density, COD removal efficiency increased due to the generation of more coagulant agents (Lakshmi and Sivashanmugam 2013). After 25 min of the treatment process, the remaining concentration of HDZ in the treatment reactor was deficient. The adsorption rate of HDZ on the flocs decreased, even though the rate of flocs generation remains constant due to the constant applied current density. However, at very low current densities, no considerable removal efficiency was observed because the amount of produced flocs was insufficient to remove all the HDZ molecules in the solution. It required more time to achieve higher HDZ removal efficiency. As seen in Fig. 5, the removal efficiency of HDZ increases from 52.9% to 67.4% by increasing the applied reaction time from 3 to 30 min. Maximum removal efficiency occurred in 25 min, at the center point of the operational parameters, including 12.5 mA cm−2 current density, solution pH of 7.0, and 27.5 mg L−1 initial HDZ concentration. However, further increasing the time to 25 provided a slight reduction of the HDZ, which would not be applied because of the high energy, and electrode consumption. Mansoorian et al. reported the optimum time for lead and zinc removal in alternating current using iron, and stainless steel rod electrodes were 30 and 40 min (Mansoorian et al. 2014).
Optimization of treatment process
As expressed in Eq. 4, the developed model was employed to determine the optional conditions for the HDZ removal. The desired inputs were selected to achieve maximum HDZ removal (˃80%) during the treatment process. Table 4 provides information on the five approaches suggested for attaining the mentioned goal. As can be seen, five solutions could result in removal efficiencies greater than 80% under the proposed conditions. Approach (V) was selected as the optimal condition due to its proximity to a neutral pH of the treated solution and low current density and reaction time.
Effect of chitosan dosage as a coagulant aid
Coagulant aids are often necessary to further enhance the coagulation performance in the water. Therefore, a new solution to contaminant removal could be using chitosan as a novel, cheap, and safe coagulant aid. Figure 6-a shows the electrocoagulation process under the optimum condition, including an initial HDZ concentration of 25 mg L−1, pH solution of 8.0, the current density of 12 mA cm−2, and reaction time of 15 min using different concentrations of chitosan as a coagulant aid. The results showed that the removal efficiencies increased from 86.5% to 99.7% by increasing the chitosan dosage from 0.01 to 0.05 g L−1. On the other hand, increasing the chitosan dosage from 0.01, 0.02, 0.03 to 0.05 g L−1 increased the electrical energy consumption (EEC) during the electrocoagulation process from 0.374, 0.412, 0.438 to 0.553 kWh m−3, respectively. The contribution of the electrocoagulation process, the adsorption process, and the hybrid process under the optimum condition is demonstrated in Fig. 6-b. Asaitambi et al. treated the distillery effluent using ozone-assisted electrocoagulation; their results showed that in optimal conditions, the EEC was 5.1 kWh m−3 (Asaithambi et al. 2012). Also, de Carvalho et al. removed methylene blue dye using electrocoagulation/banana peel adsorption process; their results indicated that under optimal conditions, the energy consumption was 1.65 Wh g−1 (de Carvalho et al. 2015). In addition, Zaidi et al. removed the doxycycline using electro-coagulation coupled electro-flotation process; their results showed that in optimal conditions (pH of 7.03, and current intensity of 5.4 mA cm−2, and reaction time of 80 min), the EEC when using NaNO3, KCl and NaCl as supporting electrolyte were 3.675, 1.455, and 1.505 kWh m−3, respectively (Zaidi et al. 2019).
According to the obtained results, at the chitosan dosage of 0.03 g L−1, the removal efficiency and EEC of 99.3% and 0.438 kWh m−3 were achieved, which is introduced as the optimal condition.
Conclusion
In this study, the HDZ removal efficiency from the polluted water was investigated by the hybrid process of electrocoagulation and adsorption using iron electrodes and chitosan. Analysis of variance (ANOVA) showed a high determination coefficient, and an adequate prediction second-order regression model was achieved. A satisfactory agreement between the experimental and predicted HDZ removal efficiency was obtained, which confirmed the accuracy and validity of the HDZ removal model. The maximum HDZ removal and electrical energy consumption were obtained in the optimum condition at 99.3% and 0.438 kWh m−3, respectively. The obtained results showed that the applied hybrid treatment process could be considered a practical alternative approach for the treatment of HDZ to achieve a higher quality aqueous solution.
References
Abbas M et al (2018) Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: a review. Sci Total Environ 626:1295–1309
Alibabaei F et al (2021) An evaluation of the efficiency of odorant removal by sodium ferrate (VI) oxidation. Measurement 179:109488
Almomani F, Bohsale RR (2020) Optimizing nutrient removal of moving bed biofilm reactor process using response surface methodology. Biores Technol 305:123059
Asaithambi P et al (2012) Ozone assisted electrocoagulation for the treatment of distillery effluent. Desalination 297:1–7
Bilińska L et al (2020) Catalytic ozonation of textile wastewater as a polishing step after industrial scale electrocoagulation. J Environ Manage 265:110502
De Carvalho H P et al (2015) Improvement of Methylene Blue removal by electrocoagulation/banana peel adsorption coupling in a batch system. Alexandria Engineering Journal. 54:777–786
El-taliawy H et al (2018) Removal of ozonation products of pharmaceuticals in laboratory Moving Bed Biofilm Reactors (MBBRs). J Hazard Mater 347:288–298
Iqbal M (2016) Vicia faba bioassay for environmental toxicity monitoring: a review. Chemosphere 144:785–802
Isik Z et al (2020) Electrocoagulation and electrooxidation pre-treatment effect on fungal treatment of pistachio processing wastewater. Chemosphere 244:125383
Jaria G et al (2020) Effect of the surface functionalization of a waste-derived activated carbon on pharmaceuticals’ adsorption from water. J Mol Liq 299:112098
Khan NA et al (2020) Recent trends in disposal and treatment technologies of emerging-pollutants-A critical review. TrAC, Trends Anal Chem 122:115744
Khandegar V (2013) Electrocoagulation for the treatment of textile industry effluent–a review. J Environ Manage 128:949–963
Kharel S et al (2020) Ozone dose dependent formation and removal of ozonation products of pharmaceuticals in pilot and full-scale municipal wastewater treatment plants. Sci Total Environ 731:139064
Kim T et al (2020) Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J Water Process Eng 33:101109
Köpping I et al (2020) Removal of pharmaceuticals from nitrified urine by adsorption on granular activated carbon. Water Research x 9:100057
Lakshmi PM, Sivashanmugam P (2013) Treatment of oil tanning effluent by electrocoagulation: Influence of ultrasound and hybrid electrode on COD removal. Sep Purif Technol 116:378–384
Lu J et al (2021) Efficient removal of Tetracycline-Cu complexes from water by electrocoagulation technology. J Clean Prod 289:125729
Luján EA et al (2020) Pre-operative anxiolysis in children through a combined pharmacological therapy with hydroxyzine and a non-pharmacological distraction technique with a clown (SONRISA): study protocol for randomised double-blind clinical trial. Trials 21:1–8
Mamo J et al (2018) Fate of pharmaceuticals and their transformation products in integrated membrane systems for wastewater reclamation. Chem Eng J 331:450–461
Mansoorian HJ et al (2014) Removal of lead and zinc from battery industry wastewater using electrocoagulation process: influence of direct and alternating current by using iron and stainless steel rod electrodes. Sep Purif Technol 135:165–175
Minas F et al (2017) Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. Chem Int 3:291–305
Pinedo-hernández J et al (2020) Removal of cypermethrin and chemical oxygen demand from livestock wastewater by electrocoagulation. Chem Eng Technol 43:211–217
Pulkka S et al (2014) Electrochemical methods for the removal of anionic contaminants from water–a review. Sep Purif Technol 132:252–271
Rosman N et al (2018) Hybrid membrane filtration-advanced oxidation processes for removal of pharmaceutical residue. J Colloid Interface Sci 532:236–260
Sandoval MA et al (2021) Arsenic and fluoride removal by electrocoagulation process: A general review. Sci Total Environ 753:142108
Sharif S et al (2021) Coagulation of metallic pollutants from wastewater using a variety of coagulants based on metal binding interaction studies. Z Phys Chem 235:467–481
Stavbar S et al (2017) Sub-and super-critical water oxidation of wastewater containing amoxicillin and ciprofloxacin. The J Supercritical Fluids 128:73–78
SyamBabu D et al (2020) Industrial wastewater treatment by electrocoagulation process. Sep Sci Technol 55:3195–3227
Tahreen A et al (2020) Role of electrocoagulation in wastewater treatment: A developmental review. J Water Process Eng 37:101440
Thomas J et al (2019) Real-World, Non-Interventional, Observational Study of Hydroxyzine Hydrochloride in Chronic Pruritus: a Prospective. Non-Comparative Study Dermatol Therapy 9:299–308
Uz Zaman Q et al (2021) Experimental modeling, optimization and comparison of coagulants for removal of metallic pollutants from wastewater. Z Phys Chem 235:1041–1053
Vepsäläinen, M. and SILLANPÄÄ, M. (2020) Electrocoagulation in the treatment of industrial waters and wastewaters. In) Advanced Water Treatment. Elsevier, pp. 1–78.
Xia Y, DAI, Q. (2018) Electrochemical degradation of antibiotic levofloxacin by PbO2 electrode: Kinetics, energy demands and reaction pathways. Chemosphere 205:215–222
Yang Y et al (2020) Insights to perfluorooctanoic acid adsorption micro-mechanism over Fe-based metal organic frameworks: Combining computational calculation with response surface methodology. J Hazard Mater 395:122686
Zaidi S et al (2019) Electro-coagulation coupled electro-flotation process: feasible choice in doxycycline removal from pharmaceutical effluents. Arab J Chem 12:2798–2809
Zhao, S., et al. (2017) Removal of antibiotics using polyethylenimine cross-linked nanofiltration membranes: relating membrane performance to surface charge characteristics. Chemical Engineering Journal.
Acknowledgements
The authors would like to express their appreciation to the student research committee of Kerman University of Medical Sciences [Grant number 400000746] for supporting the current work.
Funding
This work received a grant from the Kerman University of Medical Sciences [Grant number 400000746].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of the current paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dolatabadi, M., Kheirieh, A., Yoosefian, M. et al. Hydroxyzine removal from the polluted aqueous solution using the hybrid treatment process of electrocoagulation and adsorption; optimization, and modeling. Appl Water Sci 12, 254 (2022). https://doi.org/10.1007/s13201-022-01780-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01780-7