Abstract
Coastal areas provide the largest avenue for human settlement worldwide. Among the different sources of water, groundwater comes under the most exploited for domestic, agriculture as well as industrial purposes. The present study is aimed to evaluate the hydrochemical characteristics and quality of groundwater. A total of 132 groundwater samples were collected during post-monsoon and pre-monsoon seasons and analyzed for major and minor ions, and the results were used to evaluate water quality parameters like sodium absorption ratio, magnesium absorption ratio, soluble sodium percentage, permeability index and residual sodium carbonate to establish water quality for various utilities. Groundwater samples show acidic to alkaline nature; dominant cations reported were in the order of Na+ > Ca2+ > K+ > Mg2+ and dominant anions Cl− > HCO3− > SO42−. The hardness of water ranges from soft to very hard. The major water types noted were CaHCO3, NaHCO3, CaCl2, mixed CaNaHCO3 and NaCl. Based on electrical conductivity, total dissolved solids and total hardness majority of the samples are appropriate for consumption and domestic utilities with few exceptions. Computed parameters reveal that a majority of the groundwater samples are suitable for agricultural purposes. The Gibbs plot reveals that interaction between rock and water is the major mechanism controlling groundwater chemistry in the study area. Multivariate statistical analyses such as correlation analysis and principal component analysis were carried out for better understanding and classification of water quality and factors which control the groundwater chemistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is the most valuable and non-substitutable natural resource, which is necessary for the growth, wellness and well-being of all living organisms globally (Du Plessis 2017). Hydrosphere comprises an estimate of 1386 million km3 of water (Kibona et al. 2009; Lui et al. 2011; Du Plessis 2017). Around 97% of the total water is in oceans, and the remaining three percent is considered as freshwater. Among the freshwater, 69% is locked up in ice sheets or glaciers. Groundwater accounts for 30%, and only 0.3% of total freshwater is available on the surface as rivers, lakes and reservoirs (Cassardo and Jones 2011; Du Plessis 2017). Recent studies reveal that approximately 69% of the total withdrawal has been used for agricultural, 22% for industrial, 8% for domestic and remaining 1% for recreational purposes (Rosegrant et al. 2009; Du Plessis 2017).
The annual groundwater availability of India (Central Water Commission 2015) is about 433 Billion Cubic Meter (BCM). In Tamil Nadu, it is around 21.53 BCM that is nearly 4.98% of the total, whereas in Puducherry it is 0.160 BCM. The basic water prerequisite for human well-being and food is 50 L per capita per day and 400,000 L per year, respectively (Du Plessis 2017). During 2011, population growth has reduced 15% of the national per capita annual availability of water from 1816 to 1544 cubic meter.
Nearly 40% of the world population is confined to coastal regions. Day by day, the demand for groundwater is increasing due to population growth and intense agricultural as well as industrial activities. Overexploitation of groundwater is one of the major reasons for scarcity. Groundwater in the coastal area is susceptible to degradation due to its proximity to the sea. The salinization may happen due to natural as well as anthropogenic factors. Overexploitation of groundwater is one of the major reasons for saline water incursion in coastal areas. The quality of groundwater is highly affected by urbanization, agricultural waste, land cover, intense application of fertilizers, pesticides, utilization of wastewater for agriculture, leakage from wastewater lagoons, landfill disposal sites, septic and industrial discharge (Al-Kharabsheh 1999; Thomas 2000; Laghari et al. 2004; EL-Naqa et al. 2007; El-Saeid et al. 2011). In this scenario, the quality of groundwater has become a major concern throughout the globe. Several studies were attempted by researchers universally to establish the quality of groundwater for various purposes (Wu et al. 2015; Asare et al. 2016; Singh et al. 2015; Nag and Lahiri 2012; Lakshmanan et al. 2003; Srinivasamoorthy et al. 2009; Gopinath et al. 2014; Sridharan and Senthil Nathan 2017; Kattan 2018; Iqbal et al. 2018; Sefie et al. 2018; Reddy et al. 2019; Shamsuddin et al. 2019).
The present study has been attempted along the east coast of Tamil Nadu covering Kanchipuram and Viluppuram districts and Puducherry to establish the groundwater suitability for domestic and agricultural purposes. Net groundwater availability of Viluppuram and Kanchipuram districts is 1696 MCM and 1382.91 MCM, respectively (CGWB 2007a, 2009). Vannur block of Viluppuram district and Chithamur and Thirukazhukundram block of Kanchipuram district were categorized as critical blocks in terms of future groundwater development, whereas Lathur, Marakkanam and Kandamangalam blocks as over-exploited blocks by CGWB.
Study area
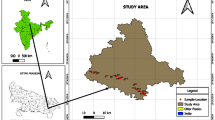
The area falls along the Coromandel Coast of Tamil Nadu as a linear stretch, which covers parts of Kanchipuram and Viluppuram districts of Tamil Nadu and the union territory of Puducherry. The area lies in between latitudes 11° 52′–12° 38′ N and longitudes 79° 38′–80° 11′ E with a total areal extension of 1570 sq.km (Fig. 1). The major rivers flowing in the study area are the River Palar and River Ponnaiyar, flowing due northern and southern parts, respectively. Geomorphologically, the majority of the study area is occupied by pediplain, coastal and alluvial plain. Flood plains, deltaic plain, uplands, structural and denudational hills were also observed to be distributed throughout the area. The area has a maximum elevation of 217 m above MSL toward the northwestern corner as an isolated hillock near Thirukazhukundram village. The maximum slope is 89° excluding this; the average slope ranges from 0.7° to 1.4°. The area experiences tropical humid climatic conditions with maximum temperature ranging from 27.9 to 36.9 °C. The area enjoys both southwest monsoon (June–September) and northeast monsoon (October–December) in which the southwest monsoon is highly erratic. In the northeast monsoon, precipitation occurs in the form of a cyclonic storm as a result of depression that develops in the Bay of Bengal. The area experiences an average annual rainfall of about 1184 mm (CGWB 2007a, b, 2009).
Geology
Geologically, the area can be classified into two litho domains, hard crystalline plutonic rocks and soft sedimentary rocks. The hard rock is characterized as charnockite of Archean age, which is noted along the western parts of the study area, and the soft rock comprises the semi-consolidated and unconsolidated sediments. The semi-consolidated sediments include sandstone and conglomerate of Upper Jurassic–Early Cretaceous age, sandstone, limestone, shaley sandstone, shelly limestone of Cretaceous age and limestone marl and shale of Paleogene age. The unconsolidated sediments are comprised of sand and silt (coastal, alluvial, eolian deposits) of recent age and form as a linear stretch toward the shoreline (Fig. 2).
Methodology
Groundwater sampling for the present study was attempted during the post-monsoon (POM) February 2014 and pre-monsoon (PRM) May 2014 seasons. A total of 66 water samples were collected from wells by considering spatial and temporal variations. All the samples were collected in one-liter polyethylene bottles which were thoroughly washed with dilute HNO3 and distilled water prior to collection. Serious care has been taken to fix the sample locations so that the samples should be a representation of the area. The samples were collected after 10–15-min pumping to remove the existing water. The sample bottles were rinsed for 2–3 times with the representative groundwater. The physicochemical parameters like EC, pH and TDS were measured in the field by the aid of pre-calibrated portable water analyzer. The sample bottles were wrapped and kept properly (at 4 °C) until analyzed.
In the laboratory, prior to analysis, all the samples were filtered using 0.45-μm Millipore filters. Standard procedures were adopted to analyze major ions as per APHA (1995). The divalent cations such as calcium and magnesium were determined by titrimetry using EDTA as a standard. Bicarbonate and chloride were analyzed by standard titration with AgNO3 and HCl, respectively. Sulfate and phosphate were determined by spectrophotometer, and sodium and potassium by flame photometry. Nitrate and fluoride were determined by ion-sensitive electrodes. The charge balance and TDS/EC ratios were used to check the analytical precision, which varies within 5–10%.
Groundwater chemical alteration and suitability analysis have been performed by using Piper plot (1944), and parameters like soluble sodium percentage (SSP), residual sodium carbonate (RSC), sodium absorption ratio (SAR), magnesium absorption ratio (MAR) and permeability index (PI) were calculated for which detailed descriptions and equations are discussed in the concerned sections. Statistical analysis is an effective tool for the characterization and interpretation of hydrochemical data sets. Statistical treatment will reduce the numerical complexity of data sets and provide significant interpretation. The relationships between the hydrochemical variable and their distribution can be easily identified using the statistical significance. Correlation analysis (CA) is used to establish the strength and interrelationship among the chemical parameters. Correlation coefficient “r” is the end product of correlation analysis, which is a numerical value ranges between + 1 and − 1. When correlation coefficient r is equal to + 1, it signifies variable with a perfect linear relationship in a positive manner, while the value of r equal to − 1 portrays a negative linear relationship between variables. When r equals zero, it infers a nonlinear relationship between variables. For the present study, factors with eigenvalues greater than or equal to 1 are considered. A total of three factors were extracted from the data sets representing post- and pre-monsoon seasons on the basis of Kaiser criterion, which was rotated according to Varimax with Kaiser normalization method. The factor loading is classified as strong (> 0.75), moderate (0.75–0.50) and week (< 0.50) based on absolute factors and their loadings (Liu et al. 2003). The statistical analysis of the groundwater samples was attempted using IBM SPSS version 23.0.
Results and discussion
Water quality for domestic purpose
pH, conductivity and total dissolved solids
The pH in the samples during POM and PRM varied between 5.1 and 7.7 and 6.2 and 8.7 with an average of 6.8 and 7.1, respectively (Table 1). The samples irrespective of seasons show acidic to alkaline nature. The permissible limit for pH in groundwater is between 6.5 and 8.5 (WHO 2004; BIS 2012). The majority of the samples from both seasons fall under permissible limit except few locations like Marakkanam, Kalapet, Kandadu and Nangadarkuppam, observed with lower pH than the permissible limit (Fig. 3a, b). These sample locations were mainly confined to the sedimentary formation, and the variation in pH might be due to anthropogenic activities like improper disposal of sewage and excess usage of fertilizers (Sarath Prasanth et al. 2012).
The electrical conductivity of water is the capacity to conduct electrical charges, which is directly proportional to the TDS in water. An increase in the concentration of dissolved ions may lead to higher conductance (Hem 1985). EC values from the samples range between 130.0 and 4350.0 and 102.0 and 8930.0 µS/cm with an average of 1461.0 and 1472.0 µS/cm during the respective seasons (Table 1; Fig. 4a, b). According to WHO (2004) and BIS (2012), the acceptable limit for EC in groundwater is 1500.0 µS/cm. Among the total, about 57.5 and 61% of post- and pre-monsoon samples were recorded with lower EC (< 1500 µS/cm), which is categorized as type I with low enrichment of salts (Sarath Prasanth et al. 2012). About 35 and 33% of the samples from both seasons fall within the range of 1500 µS/cm and 3000 µS/cm, which is classified under type II with moderate salt enrichment. About 7.5 and 6.0% samples from both seasons fall under type III (> 3000 µS/cm) with maximum enrichment of salts. The higher value of EC in the area is accredited by saline sources like seawater, salt pans, aquaculture and incursion of pollutants from anthropogenic activity. Kuvathur, Manamai, Viludamangalam, Perumbedu and Alathur are the areas with higher values of EC.
Total dissolved solids are the measure of all organic as well as inorganic substances present in water. Higher TDS values will affect the taste of drinking water (Nag and Suchetana 2016). The acceptable limit for TDS is 1000 mg/L as per WHO (2004) and BIS (2012). About 75.8 and 81.8% of the samples from both seasons are well within the permissible limit, while 24.2 and 18.2% samples exceed the limit. The TDS in samples varies from 53.9 to 4710.0 and 101.0 to 1760.0 mg/L with an average of 731.0 and 776.6 mg/L in POM and PRM correspondingly (Table 1; Fig. 5a, b). Groundwater suitability with respect to TDS for drinking and agricultural purposes is mentioned in Table 2. According to the limits, 75.7 and 81.8% of samples are suitable for drinking and agricultural purpose, whereas 24.2 and 18.2% of the samples are suitable only for agricultural purposes during both seasons. Higher TDS in the study area specifies improper sewage disposal, mineral dissolution and concentration of salts from point sources as discussed earlier.
Major cations
Calcium in samples ranges between 3.0 and 302.0 and 4.0 and 140.0 mg/L with averages 43.10 and 49.90 mg/L during POM and PRM, respectively (Table 1). The highest desirable limit for Ca2+ as per WHO (2004) and BIS (2012) standards is 75.0 mg/L. Among the total, 1.50% of PRM samples exceed the maximum permissible limit (Fig. 6a, b). Higher values were recorded near Alathur, Sedarpattu, Manamai, Manikuppam, Agaram Perumbedu and Nelvay that confined to hard rock terrain with few exceptions also in the sedimentary environment. The Ca2+ in groundwater might have been derived from litho units as a result of rock–water interaction and mineral dissolution. The magnesium in the samples ranges between 5.0 and 87.0 and between 2.40 and 128.0 mg/L with an average of 23.70 and 26.30 mg/L during POM and PRM, respectively (Table 1). The maximum desirable limit for Mg2+ is 30.0 mg/L (WHO 2004; BIS 2012), and about 1.50% of PRM samples exceed the limit. The higher values of Mg2+ are observed in Perumbedu and Manamai areas (Fig. 7a, b).
Excess sodium in groundwater may result in salty taste. It often occurs naturally in groundwater due to its greater solubility. Sodium consumption at a regular level is not harmful; increased intake may result in serious health hazards like hypertension, heart disease and kidney stone (Nag and Suchetana 2016). The major sources of sodium in groundwater include saltwater intrusion, sewage effluent, dissolution of salt from sodium-rich rocks, and infiltration from contaminated surface sources like salt pans, industrial and irrigational sites. The maximum permissible limit of Na+ in groundwater as per the standards of WHO (2004) and BIS (2012) is 200 mg/L. Sodium concentration in the samples from the study area varies from 8.0 to 288.0 and 4.0 to 300.0 mg/L with an average of 71.50 and 90.0 mg/L during POM and PRM, respectively. All the samples irrespective of seasons were noted within the limit (Table 1). The Na+ in groundwater is derived mainly from weathering of feldspars and from saline sources like salt pans and seawater. The potassium in the groundwater varies from ND to 420.0 and 0.80 to 355.0 mg/L in both seasons (Fig. 8a, b). Excess use of fertilizers for agricultural purposes in the study area might be the reason for higher values.
Major anions
The concentration of bicarbonate controls the alkalinity of groundwater. Bicarbonate concentration in the samples varies from 40.0 to 591.70 and 40.0 to 560.0 mg/L with an average of 243.90 and 253.60 mg/L during POM and PRM, respectively (Table 1; Fig. 9a, b). Higher bicarbonate in samples might be due to mineral dissolution. The sulfate in groundwater is an essential nutrient for plants; excess sulfate may affect the salinity as well as the hardness of water (Nag and Suchetana 2016). The sulfate concentration ranges from ND to 6.30 and 5.30 mg/L in the respective seasons (Table 1). As per WHO, the highest desirable limit of sulfate in water is 200.0 mg/L, which indicates that all the samples fall within the limit.
Chloride concentration in samples varied between 20.0 and 660.0 and between 15.0 and 2020.0 mg/L in both the seasons. According to WHO and BIS, the desirable limit of chloride in water is 250.0 mg/L and the maximum permissible limit is 1000.0 mg/L. Higher Cl− was confined to a sedimentary environment except few locations confined to the northern part of the study area signifying saline sources like seawater, salt pans and industrial waste (Fig. 10a, b).
The phosphate in groundwater is mainly due to the excessive use of fertilizers during agricultural practices (Amadi et al. 2012). It may also be derived from apatite mineral confined to charnockite present in the study area (Chidambaram 2000). The phosphate in the samples ranges from ND to 4.90 and ND to 7.30 mg/L with an average of 0.40 and 2.20 mg/L during POM and PRM, respectively. Higher concentrations of PO43− were reported from northern, central and southern parts of the study area during PRM season which might be due to the influence of agricultural activities (Kortatsi et al. 2007). Major nitrate sources to groundwater are mainly due to leaching of chemical or synthetic fertilizers, nitrogenous organic waste, septic tank, sewage disposal and bacterial action (Hem 1991; Srinivasamoorthy 2004). Nitrate concentration of the samples ranges from ND to 56.0 and 0.20 to 82.50 mg/L with an average of 9.88 and 12.19 mg/L during POM and PRM, respectively. Elevated nitrate was observed along the eastern part of the study area during the PRM season, which might have been derived from fertilizers used during agricultural purposes (Mantelin and Touraine 2004). The fluoride in the groundwater samples varies from 0.20 to 1.60 and 0.04 to 0.65 mg/L with an average of 0.68 and 0.10 mg/L during POM and PRM correspondingly. Elevated F values were reported from the northwestern part of the study area during the POM season, indicating the release of fluoride ions from the country rock (Moncaster et al. 2000).
The chemical analysis of dissolved ions in the groundwater samples reveals that majorities are within the permissible limit with some exceptions. However, drastic changes in the concentration of certain dissolved parameters were observed, which might be due to the rise in the water table during the post-monsoon period, which may dissolve more salts from the soil materials and thereby increase the salinity of groundwater after the monsoon (Ramesam 1982; Ballukraya and Ravi 1999; Rajmohan and Elango 2003).
Total hardness (TH)
Water possesses two specific types of hardness, temporary and permanent; the former is due to the presence of dissolved bicarbonates such as CaHCO3 and MgHCO3, which will remove when the water is boiled, and the latter is due to the presence of CaSO4, MgSO4, CaCl2 and MgCl2, which gets removed by ion exchange process (Nag 2014). The hardness of the samples is obtained by the following equation:
About 4.50 and 7.60% of samples are soft; 36.40 and 25.80% belong to moderate hard; 43.90 and 42.40% samples belong to hard, whereas the remaining 15.20 and 24.20% encompass very hard during POM and PRM, respectively, which is confined mainly in the hard crystalline rock terrain (Table 2; Fig. 11a, b)
Piper diagram
Hydrochemical facies, as well as hydrochemical evolution of groundwater, can be deciphered by the help of piper trilinear diagram (Piper 1944). The diagram unveils the chemical relationship between the groundwater samples. The diagram represents cations and anions in separate ternary plots, and the ternary plots were extrapolated to a diamond field in which different water types were marked. Hydrochemical facies analysis is a valuable tool for establishing the flow pattern and chemical evolution of groundwater masses (Srinivasamoorthy et al. 2010). The groundwater samples spread within CaHCO3, NaHCO3, CaCl, mixed CaNaHCO3 and NaCl types, irrespective of seasons. The samples that fall in the CaHCO3 field represent the recharge water with temporary hardness, having more Ca2+, Mg2+ and HCO3 which are released from the litho unit during the percolation of water (Schoeller 1965). The samples that fall in the CaNaHCO3 field indicate the dominance of ion exchange which results in permanent hardness. The samples in the NaCl field indicate saline water, whereas the fields 5 and 6 indicate mixing (Fig. 12).
Gibbs diagram
The hydrochemical processes like precipitation, evaporation and rock–water interaction which involve controlling the groundwater chemistry can be recognized by the aid of Gibbs diagram (1970). The Gibbs diagram is prepared separately for anions and cations by plotting the TDS in the logarithmic axis against the Gibbs ratio in the linear axis. The Gibbs ratio is obtained by using the formula:
The Gibbs plot (Fig. 13a, b) suggests that rock–water interaction which involves chemical weathering, dissolution and precipitation influenced the water chemistry to a considerable extent during the pre- and post-monsoon seasons (Krishna Kumar et al. 2014). However, some points fall near the zone of evaporation, which indicates the minor influence of evaporation in controlling the water chemistry. It can be inferred that shifting of sample points toward evaporation dominance might be due to contamination of saline sources which release more Na+ and Cl− to the groundwater.
Irrigation water quality
Irrigation is considered as the basic requisite for agriculture; its deficiencies are the most controlling limits on the increase in agricultural production. In this scenario, it is very essential to assure the irrigation water quality as it may directly affect the crop yield and fertility of the soil. Certain water quality parameters were calculated to establish the water quality for irrigational purposes. For these calculations, all ionic concentrations were taken in meq/L. This includes soluble sodium percentage (SSP), residual sodium carbonate (RSC), sodium absorption ratio (SAR), magnesium absorption ratio (MAR) and permeability index (PI). The values were compared along with the standards to establish irrigational suitability of groundwater (Table 3).
Sodium absorption ratio (SAR)
It defines the excess sodium or alkali hazard in groundwater. It is the ratio between sodium with respect to calcium and magnesium (Iqbal et al. 2012; Haritash et al. 2014; Sridharan and Senthil Nathan 2017). Groundwater with high sodium may affect the infiltration capacity of the soil which ultimately results in the formation of alkaline soil. SAR values were calculated using the given formula (Richards 1954):
The values range from 0.1 to 10.8 and 0.1 to 15.4 during the post- and pre-monsoon seasons; all samples of both seasons fall within excellent to good category. Wilcox diagram was created by plotting the SAR and EC values (Fig. 14). In the Wilcox diagram, majority of the samples from POM (45%) and PRM (46%) fall in C3–S1, which indicates that salinity is high and sodium is low in concentration and the water can be used under favorable conditions for salt-tolerant to semi-tolerant crops (Alaya et al. 2013; Tiwari and Singh 2014). About 30% of POM and 25% of PRM samples are representing C1–S1 and C2–S1 classes which indicate low sodium and low to medium salinity, which is considered as good water for irrigation purposes. The samples that fall in C3–S2 class indicate that the water possesses high salinity and medium sodicity. Usage of this water in fine-grained soils with controlled drainage may result in salt accumulation in root zones of crops and eventually leads to soil clogging and salinity (Srinivasamoorthy et al. 2011; Vasanthavigar et al. 2012). Minor representations were also noted in C3–S3, C3–S4, C4–S1 and C4–S2 classes; it shows samples with high salinity and medium to high sodicity; such types of water are not suitable for irrigation on low-permeable clayey soil (Singh et al. 2015). In regions with high salinity, plants with more salt tolerance level can be selected, and for considerable leaching, water should be provided in surplus quantity (Karanth 1989; Singh et al. 2012).
Soluble sodium percentage (SSP)
SSP is expressed in Na%, which is an important criterion to categorize irrigation water. High sodium in irrigation water may lead to the exchange of sodium in water for calcium and magnesium ions, which will reduce the internal drainage capacity of the soil (Collins and Jenkins 1996; Singh et al. 2013). SSP is calculated using the equation:
The SSP values range from 4.0 to 95.0 and 4.0 to 97.0 with an average of 44.0 and 47.0 during both the seasons, and the results were plotted against EC in the Wilcox diagram to classify the groundwater. According to the classification, 25.80 and 30.30% of samples represent excellent to good category; 30.30 and 28.80% fall within good to permissible limit; 21.20 and 19.70% are permissible to doubtful; 13.60 and 16.70% are doubtful to useful; and 9.10 and 4.50% are unsuitable for agricultural purposes in the respective seasons. Samples from Venganpakkam, Nanakalmedu, Manamai, Valudavur, Kuvathur, Viludamangalam and Perumbedu areas are found unsuitable (Fig. 15).
Permeability Index (PI)
Soil permeability is highly affected by prolonged irrigational activities. Doneen (1962) has developed a classification of groundwater for irrigational purposes according to the permeability index. It is calculated by the formula:
The groundwater classification of samples based on this parameter (Doneen 1962) is shown in Fig. 16. Among the total samples, majority are falling in class I as well as in class II, which comes under the category of good for agricultural purposes, whereas the samples from Mangalam, Viludamangalam, Koonimedu, Kandamangalam, Marakkanam, Kolathur, Nagar, Thenpattinam, Venganpakkam, Virapuram and Munnur from respective seasons fall within class III, which is not suitable for irrigation with 25% of maximum permeability.
Magnesium absorption ratio (MAR)
Magnesium and calcium in water tend to maintain a state of equilibrium; this may lead to the degradation of the soil quality and crop yield, which results in the development of alkali soil (Paliwal 1972). MAR is determined by the formula:
Based on this, the water is broadly classified into two categories. Groundwater with MAR value < 50 is suitable and > 50 is unsuitable for agricultural purposes. MAR values in the study area range from 8.0 to 76.0 and 7.0 to 73.0 with an average of 49.0 and 47.0 in POM and PRM, respectively. Among the total samples, 45% of post-monsoon and 50% of post-monsoon samples are having MAR values > 50 and indicate that the water is unsuitable for agricultural purposes, and long-term usage of such water may affect the crop yield (Fig. 17a, b).
Residual sodium carbonate (RSC)
Excess concentration of carbonate and bicarbonate in irrigation water leads to the accumulation of calcium and magnesium ions in the soil, which may tend to increase the proportion of sodium ions. High RSC values in water may enhance the adsorption of sodium in soil (Eaton 1950). RSC index is used to establish the groundwater suitability for agricultural purposes in the case of clayey soil. RSC in groundwater is obtained by the formula:
Based on the RSC value, groundwater is classified as suitable, marginally safe and unsuitable for agricultural purposes. In the study area, 77 and 65% of POM and PRM samples are safe for agricultural purposes with RSC values < 1.25, 12 and 14% of the samples are marginally suitable with RSC values within 1.25–2.50, whereas the rest 11 and 21% of the water samples from both seasons are unsuitable for agricultural purposes with RSC values > 2.50. Mangalam, Marakkanam, Valudavur, Kandamangalam and Thenpattinam are some of the areas with high RSC values (Fig. 18a, b).
Multivariate statistical analysis
The hydrochemical data sets were subjected to multivariate statistical analysis such as Pearson correlation and principal component analysis (PCA) for better understanding.
Pearson correlation matrixes of the various parameters of groundwater samples during both seasons were calculated and are displayed in Tables 4 and 5. The correlation matrix of the post-monsoon EC shows a good positive correlation with Na+, Cl−, Mg2+ and HCO3−, which show the mobility of ions. The pre-monsoon samples elucidate positive correlations of Ca2+, Mg2+, Cl− and SO4 with EC and TDS, which indicates the impact of multiple processes such as ion exchange, mineral dissolution, saline water intrusion and anthropogenic activities like use of fertilizers and sewage disposal over the water chemistry. The very high positive correlation between EC and TDS during the pre-monsoon season specifies that groundwater contains only charged soluble components.
The principal component analysis extracted four factors from the data sets rotated according to Varimax with Kaiser normalization. The results of PCA analysis such as factor loadings, commonalities, percentage of variance and cumulative percentage of variance are summarized in Table 6. The PCA analysis explains a total variance of about 72.06 and 70.85% during POM and PRM, respectively.
In post-monsoon, Factor 1 exhibits 35.82% of the variance with higher loadings of EC, TDS, Ca2+, Mg2+, Cl− and SO42−. Factor 2 shows 15.45% of the variance with positive loadings of Na+, K+, PO43− and NO3. Factor 3 displays 10.54% of the variance with the highest loadings of PO43−, which might have been derived from the agricultural activities from the study area. The synthetic fertilizers used for agriculture possess phosphorous (Srinivasamoorthy et al. 2014). Factor 4 illustrates 10.24% of the variance with elevated loadings of pH and HCO3−, which denotes the same processes as mentioned earlier. In pre-monsoon, Factor 1 displays 21.85% variance with higher loadings of EC, TDS, Ca2+, Mg2+ and Cl−. Factor 2 shows 17.43% variance with positive loadings of Na+, Cl− and SO42−. Factor 1 represents for ion exchange and mineral dissolution, and Factor 2 stands for the saline water intrusion along the coastal areas. Factor 3 shows 16.75% variance with loadings of K+, PO43− and NO3−, which points toward agricultural activities in the study area. Factor 4 contributes to 14.80% of the variability, keeps the highest loadings of pH and HCO3− and implies a natural process of accumulation of H+ ions into the system along with HCO3−.
Conclusion
The groundwater quality along the coastal tracts of Kanchipuram and Viluppuram districts of Tamil Nadu and Puducherry has been analyzed to establish its suitability for domestic as well as agricultural purposes. The analytical results reveal that dominant cations and anions are in the sequence Na+ > Ca2+ > K+ > Mg2+ and Cl− > HCO3− > SO42−. Piper plot unveils that the majority of the samples spread within CaHCO3, NaHCO3, CaCl, mixed CaNaHCO3 and NaCl types irrespective of seasons and show two distinct evolutionary paths. The pH, electrical conductivity and TDS values of the samples are within permissible limit with some exception, which crosses the limits. The total hardness of the study area varied from soft to very hard. Overall, the groundwater in the study area was found to be suitable for drinking purposes except for some places.
Groundwater suitability for the agricultural purposes was accessed from SAR, SSP, PI and MAR. Based on SAR, the majority of the samples in the area have low sodium hazard except for few samples with moderate to high sodium, and in terms of salinity hazard, the samples in the area have low to very high salinity. Soluble sodium percentage values indicate that the majority of the samples are suitable for agricultural purposes except for a few samples which are unsuitable. RSC values of few samples indicate that long-term usage of the water may affect the crop yield. Based on MAR, groundwater at some places is unsuitable for agricultural purposes. The Gibbs plot was employed to identify the process and mechanism involved in controlling the water chemistry; it is inferred that rock–water interaction, i.e., chemical weathering and mineral dissolution, is the major factor that controls the water chemistry. The multivariate statistical analysis reveals that groundwater quality in the study area is affected by both natural and anthropogenic sources. Anthropogenic activities such as sewage disposal, intense use of fertilizers, aquaculture and salt pan activities give direct stress to the groundwater quality. Overdrafting of groundwater along the coastal area leads to inland migration of saline water into the coastal aquifers. Proper awareness and regular monitoring have to be done to protect the groundwater from further deterioration.
References
Al-Kharabsheh AA (1999) Influence of urbanization on water quality at Wadi Kufranja basin (Jordan). J Arid Environ 43(1):79–89. https://doi.org/10.1006/jare.1999.0534
Alaya MB, Saidi S, Zemni T, Zargouni F (2013) Suitability assessment of deep groundwater for drinking and irrigation use in the Djeffara aquifers (Northern Gabes, south-eastern Tunisia). Environ Earth Sci 71(8):3387–3421. https://doi.org/10.1007/s12665-013-2729-9
Amadi AN, Olasehinde PI, Yisa J, Okosun EA, Nwankwoala HO, Alkali YB (2012) Geostatistical assessment of groundwater quality from coastal aquifers of Eastern Niger Delta, Nigeria. Geophys J R Astron Soc 2(3):51–59
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. Washington, p 1467
Asare R, Sakyi PA, Fynn OF, Osiakwan GM (2016) Assessment of groundwater quality and its suitability for domestic and agricultural purposes in parts of the Central Region, Ghana. West African J Appl Ecol 24(2):67–89
Ballukraya PN, Ravi R (1999) Characterization of groundwater in the unconfined aquifers of Chennai City, India; Part I: Hydrogeochemistry. J Geol Soc India 54:1–11
Bureau of Indian Standards (BIS) (2012) Indian standard drinking water specification (second revision) BIS 10500:2012, New Delhi
Cassardo C, Jones JAA (2011) Managing water in a changing world. Water 3:618–628. https://doi.org/10.3390/w3020618
Central Ground Water Board (CGWB) (2007a) District groundwater brochure Kancheepuram district. http://cgwb.gov.in/district_profile/tamilnadu/kancheepuram.pdf. Accessed 15 Oct 2017
Central Ground Water Board (CGWB) (2007b) Groundwater Brochure of Puducherry Region U.T of Puducherry, pp 1–27. http://www.cgwb.gov.in/District_Profile/Puduchery/Puducherry.pdf. Accessed 15 Oct 2017
Central Ground Water Board (CGWB) (2009) District groundwater brochure Villupuram district, Tamil Nadu. Technical report series. http://cgwb.gov.in/district_profile/tamilnadu/villupuram.pdf. Accessed 15 Oct 2017
Central Water Commission (2015) Water and related statistics. http://www.cwc.gov.in/main/downloads/Water%20&%20Related%20Statistics%202015.pdf. Accessed 17 Oct 2017
Chidambaram S (2000). Hydrogeochemical studies of groundwater in Periyar district, Tamilnadu, India. Ph.D. thesis, Department of Geology, Annamalai University
Collins R, Jenkins A (1996) The impact of agricultural land use on stream chemistry in the Middle Hills of the Himalayas, Nepal. J Hydrol 185(1–4):71–86. https://doi.org/10.1016/0022-1694(95)03008-5
Davis SN, De Wiest RJM (1966) Hydrogeology, vol 463. Wiley, NewYork
Doneen LD (1962) The influence of crop and soil on percolating water. In: Proceedings of the 1961 Biennial conference on groundwater recharge, pp 156–163
Du Plessis A (2017) Global water availability, distribution and use. Water. https://doi.org/10.1007/978-3-319-49502-6_1
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 69(2):123–134. https://doi.org/10.1097/00010694-195002000-00004
El-Naqa A, Al-Momani M, Kilani S, Hammouri N (2007) Groundwater deterioration of shallow groundwater aquifers due to overexploitation in Northeast Jordan. CLEAN Soil Air Water 35(2):156–166. https://doi.org/10.1002/clen.200700012
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090. https://doi.org/10.1126/science.170.3962.1088
Gopinath S, Srinivasamoorthy K, Prakash R (2014) Hydrochemical investigations for identification of groundwater salinization sources in Nagapattinam and Karaikal regions. Southern India. Environ Geo Chem Acta 1(2):153e160
Haritash AK, Gaur S, Garg S (2014) Assessment of water quality and suitability analysis of River Ganga in Rishikesh, India. Appl Water Sci 6(4):383–392. https://doi.org/10.1007/s13201-014-0235-1
Hem JD (1985) Study and interpretation of the chemical characteristics of natural waters, 3rd edn. USGS Water Supply Paper, vol 2254, pp 117–120
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water, 3rd edn. US Geological Survey Water-Supply
Iqbal H, Inam A, Bakhtiyar Y, Inam A (2012) Effluent quality parameters for safe use in agriculture. Water Qual Soil Manag Irrig Crops. https://doi.org/10.5772/31557
Iqbal J, Nazzal Y, Howari F, Xavier C, Yousef A (2018) Hydrochemical processes determining the groundwater quality for irrigation use in an arid environment: the case of Liwa Aquifer, Abu Dhabi, United Arab Emirates. Groundw Sustain Dev 7:212–219. https://doi.org/10.1016/j.gsd.2018.06.004
Karanth KR (1989) Groundwater assessment development and management. Tata McGraw-Hill Publ. Com. Ltd., New Delhi
Kattan Z (2018) Using hydrochemistry and environmental isotopes in the assessment of groundwater quality in the Euphrates alluvial aquifer, Syria. Environ Earth Sci. https://doi.org/10.1007/s12665-017-7197-1
Kibona D, Kidulile G, Rwabukambara F (2009) Environment, climate warming and water management. Trans Stud Rev 16(2):484–500. https://doi.org/10.1007/s11300-009-0084-z
Kortatsi BK, Tay CK, Anornu G, Hayford E, Dartey GA (2007) Hydrogeochemical evaluation of groundwater in the lower Offin basin, Ghana. Environ Geol 53(8):1651–1662. https://doi.org/10.1007/s00254-007-0772-0
Krishna Kumar S, Bharani R, Magesh NS, Godson PS, Chandrasekar N (2014) Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl Water Sci 4(4):341–350. https://doi.org/10.1007/s13201-013-0148-4
Laghari A, Chandio SN, Khuhawar MY, Jahangir TM, Laghari SM (2004) Effect of wastewater on the quality of ground water from southern parts of Hyderabad City. J Biol Sci 4(3):314–316. https://doi.org/10.3923/jbs.2004.314.316
Lakshmanan E, Kannan R, Senthil Kumar M (2003) Major ion chemistry and identification of hydrogeochemical processes of ground water in a part of Kancheepuram district, Tamil Nadu. India. Environ Geosci 10(4):157–166. https://doi.org/10.1306/eg100403011
Liu W, Li X, Shen Z, Wang D, Wai OW, Li Y (2003) Multivariate statistical study of heavy metal enrichment in sediments of the Pearl River Estuary. Environ Pollut 121(3):377–388. https://doi.org/10.1016/s0269-7491(02)00234-8
Lui J, Dorjderem A, Fu J, Lei X, Lui H, Macer D, Qiao Q, Sun A, Tachiyama K, Yu L, Zheng Y (2011) Water ethics and water resource management. Ethics and Climate Change in Asia and the Pacific (ECCAP) Project, Working Group 14 Report. UNESCO Bangkok
Mantelin S, Touraine B (2004) Plant growth-promoting bacteria and nitrate availability. Impacts on root development and nitrate uptake. J Exp Bot. https://doi.org/10.1093/jxb/erh010
Moncaster S, Bottrell S, Tellam J, Lloyd J, Konhauser K (2000) Migration and attenuation of agrochemical pollutants: insights from isotopic analysis of groundwater sulphate. J Contam Hydrol 43(2):147–163. https://doi.org/10.1016/s0169-7722(99)00104-7
Nag SK (2014) Evaluation of hydrochemical parameters and quality assessment of the groundwater in Gangajalghati Block, Bankura District, West Bengal, India. Arabian J Sci Eng 39(7):5715–5727. https://doi.org/10.1007/s13369-014-1141-4
Nag SK, Lahiri A (2012) Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Dwarakeswar watershed area, India. Am J Clim Change 01(04):217–230. https://doi.org/10.4236/ajcc.2012.14019
Nag SK, Suchetana B (2016) Groundwater Quality and its Suitability for Irrigation and Domestic Purposes: a Study in Rajnagar Block, Birbhum District, West Bengal, India. J Earth Sci Clim Change 7:337. https://doi.org/10.4172/2157-7617.1000337
Paliwal KV (1972) Irrigation with saline water) [Z]. Monogram No. 2 (new series). IARI, New Delhi, p 19
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25(6):914. https://doi.org/10.1029/tr025i006p00914
Raghunath IIM (1987) Groundwater, 2nd edn. Wiley Eastern Ltd., New Delhi, India, pp 344–369
Rajmohan N, Elango L (2003) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol. https://doi.org/10.1007/s00254-004-1012-5
Ramesam V (1982) Geochemistry of groundwater from a typical hard rock terrain. J Geol Soc India 23:201–204
Reddy BM, Sunitha V, Prasad M, Reddy YS, Reddy MR (2019) Evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Anantapur, Andhra Pradesh State, South India. Groundw Sustain Dev 9:100262. https://doi.org/10.1016/j.gsd.2019.100262
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Soil Sci 78(2):154. https://doi.org/10.1097/00010694-195408000-00012
Rosegrant MW, Ringler C, Zhu T (2009) Water for agriculture: maintaining food security under growing scarcity. Annu Rev Environ Resour 34(1):205–222. https://doi.org/10.1146/annurev.environ.030308.090351
Saeid MHE, Turki AMA, Wable MIA, Nasser GA (2011) Evaluation of pesticide residues in Saudi Arabia ground water. Res J Environ Sci 5(2):171–178. https://doi.org/10.3923/rjes.2011.171.178
Sarath Prasanth SV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci 2(3):165–175. https://doi.org/10.1007/s13201-012-0042-5
Sawyer GN, McCarty DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw Hill, New York, p 518
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and development. UNESCO
Sefie A, Aris AZ, Ramli MF, Narany TS, Shamsuddin MKN, Saadudin SB, Zali MA (2018) Hydrogeochemistry and groundwater quality assessment of the multilayered aquifer in Lower Kelantan Basin, Kelantan, Malaysia. Environ Earth Sci 77(10):397. https://doi.org/10.1007/s12665-018-7561-9
Shamsuddin MKN, Sulaiman WNA, Ramli MFB, Kusin FM (2019) Geochemical characteristic and water quality index of groundwater and surface water at Lower River Muda Basin, Malaysia. Arab J Geosci 12(9):309. https://doi.org/10.1007/s12517-019-4449-2
Singh AK, Mondal GC, Singh TB, Singh S, Tewary BK, Sinha A (2012) Hydrogeochemical processes and quality assessment of groundwater in Dumka and Jamtara districts, Jharkhand, India. Environ Earth Sci 67(8):2175–2191. https://doi.org/10.1007/s12665-012-1658-3
Singh AK, Raj B, Tiwari AK, Mahato MK (2013) Evaluation of hydrogeochemical processes and groundwater quality in the Jhansi district of Bundelkhand region, India. Environ Earth Sci 70(3):1225–1247. https://doi.org/10.1007/s12665-012-2209-7
Singh S, Raju NJ, Ramakrishna C (2015) Evaluation of groundwater quality and its suitability for domestic and irrigation use in parts of the Chandauli-Varanasi region, Uttar Pradesh, India. J Water Resour Prot 07(07):572–587. https://doi.org/10.4236/jwarp.2015.77046
Sridharan M, Senthil Nathan D (2017) Groundwater quality assessment for domestic and agriculture purposes in Puducherry region. Appl Water Sci 7(7):4037–4053. https://doi.org/10.1007/s13201-017-0556-y
Srinivasamoorthy K (2004) Hydrogeochemistry of Groundwater in Salem District, Tamil Nadu, India; Unpublished Ph.D. Thesis, Annamalai University, India
Srinivasamoorthy K, Nanthakumar C, Vasanthavigar M, Vijayaraghavan K, Rajivgandhi R, Chidambaram S, Vasudevan S (2009) Groundwater quality assessment from a hard rock terrain, Salem district of Tamilnadu, India. Arab J Geosci 4(1–2):91–102. https://doi.org/10.1007/s12517-009-0076-7
Srinivasamoorthy K, Vijayaraghavan K, Vasanthavigar M, Sarma S, Chidambaram S, Anandhan P, Manivannan R (2010) Assessment of groundwater quality with special emphasis on fluoride contamination in crystalline bed rock aquifers of Mettur region, Tamilnadu, India. Arab J Geosci 5(1):83–94. https://doi.org/10.1007/s12517-010-0162-x
Srinivasamoorthy K, Vasanthavigar M, Vijayaraghavan K, Sarathidasan R, Gopinath S (2011) Hydrochemistry of groundwater in a coastal region of Cuddalore district, Tamilnadu, India: implication for quality assessment. Arab J Geosci 6(2):441–454. https://doi.org/10.1007/s12517-011-0351-2
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M, Sarma VS (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu, India. J King Saud Univ Sci 26(1):37–52. https://doi.org/10.1016/j.jksus.2013.08.001
Thomas MA (2000) The effect of residential development on ground-water quality near Detroit, Michigan. J Am Water Resour Assoc 36(5):1023–1038. https://doi.org/10.1111/j.1752-1688.2000.tb05707.x
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York
Tiwari A, Singh A (2014) Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. J Geol Soc India. https://doi.org/10.1007/s12594-014-0045-y
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2012) Identification of groundwater contamination zones and its sources by using multivariate statistical approach in Thirumanimuthar sub-basin, Tamil Nadu. India. Environ Earth Sci 68(6):1783–1795. https://doi.org/10.1007/s12665-012-1868-8
Wilcox LV (1955) Classification and use of irrigation waters. USDA, Circular 969, Washington DC, USA
World Health Organization (2004) Guidelines for drinking-water quality: recommendations, vol 1. World Health Organization, Geneva
Wu H, Chen J, Qian H, Zhang X (2015) Chemical characteristics and quality assessment of groundwater of exploited aquifers in Beijiao water source of Yinchuan, China: a case study for drinking, irrigation, and industrial purposes. J Chem 2015:1–14. https://doi.org/10.1155/2015/726340
Acknowledgements
The author would like to express his sincere gratitude toward the funding authority University Grants Commission for providing (UGC)-BSR fellowship to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, A.F., Srinivasamoorthy, K. & Rabina, C. Hydrochemical characteristics and quality assessment of groundwater along the coastal tracts of Tamil Nadu and Puducherry, India. Appl Water Sci 10, 74 (2020). https://doi.org/10.1007/s13201-020-1158-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-1158-7