Abstract
Hexavalent chromium was adsorbed from aqueous solution with three prepared and characterized adsorbents, namely goethite (G), activated carbon (AC) and their composite (GAC). The goethite particle was synthesized using the precipitation methods, and activated carbon was prepared from the stem bark of Daniellia oliveri tree and composite in a ratio of 1:5 goethite–activated carbon. The adsorption capacities of G, AC and GAC for Cr(VI) are 6.627, 5.455 and 6.354 mg/g with 0.02 g adsorbent within contact time of 60, 180 and 30 min for G, AC and GAC, respectively, for Cr(VI) adsorption at optimum pH of 3. The isotherm studied was best explained by Langmuir adsorption isotherm and fitted with the pseudo-second-order kinetic model. Desorption studies showed that 1.0 M HNO3 was a better desorbing agent than 0.1 M HNO3, 0.1 M HCl and 1.0 M HCl. Chromium was most desorbed (94.60% in Cr//G using 1 M HNO3). The result obtained revealed that goethite and activated carbon produced are favourable adsorbents and the composite of the two adsorbents gives a more favourable, economical and affordable adsorbent for the clean-up of heavy metal contamination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The level of contamination of water bodies by textile, steel, paints and electroplating industries is on the alarming rate in developing countries all over the world due to rapid industrialization. High concentration of metal ions has been connected to birth defects, cancer of the digestive tract and lungs, skin lesions, retardation leading to disabilities, liver and kidney damage, and a lot of serious health effect (Jain et al. 2009). It is of utmost importance to make efforts to reduce the wastewater generation in order to provide clean water that meets our teeming population needs (Franca et al. 2015).
In the search for ways of tackling these environmental issues, numerous techniques available in this concern have been reported (Zouboulis et al. 2009; Punzi et al. 2015; Asghar et al. 2015). These methods include deposition, chemical precipitation, biological treatment, advanced oxidation process, evaporation, ion exchange, reverse osmosis, electrodeposition, solvent extraction, use of adsorbents and phytoextraction. A number of listed factors affect efficiency of the processes, and adsorption has demonstrated to be an effective technique. Most of these methods have some imperfections such as high cost of operation, high required investment and disposal of metal sludge; therefore, efforts have been made to develop cheap materials for removing pollutants from aquatic environment (Dizadji and Abootalebi 2011; Malakootan et al. 2009; Shen et al. 2009).
The advancement in using nanoparticles has solved some of the problems in this regard (Ambashta and Sillanpaa 2010), having appropriate adsorption surface; nanoparticles possess unique characteristics as they have very high surface area-to-volume ratio. This provides a tremendous driving force for diffusion, especially at high temperatures (Faraji et al. 2010; Xu et al. 2012). Because of the largest pore size of activated carbon and the greater surface area per weight of nanoparticles such as goethite and hematite, researchers have continued to improve its application as one of the fastest growing areas in environmental application such as wastewater treatment, as compositing these various adsorbents enhances more effectiveness of adsorption process and cost-friendly. In the clean-up of wastewater, activated carbon from different agricultural waste materials has been used for purification, decolourization and removal of toxic heavy metal ions.
The need to look for a cost-effective material to be used for the removal of pollutant from the environment has been the great impetus for the use of Daniellia oliveri stem bark for the preparation of activated carbon. The activated carbon composite with goethite particles will be used in the removal of toxic metals from aqueous solution.
Experimental
Collection and sample preparation
Preparation of activated carbon
Activated carbon was produced from the stem bark of Daniellia oliveri tree that grows around the Chemical Engineering Laboratory Building, University of Ilorin. The tree bark was washed, sun-dried and air-dried. This was followed by carbonization and activation with 75% H3PO4 in the ratio H3PO4/char of 1.0.
Synthesis of goethite particle
Goethite particle was prepared using the modified method of Schwertmann and Cornell (1991) with slight modification under N2 atmosphere. Hundred millilitres of 1 M Fe(NO3)3 solution was added rapidly to 180 ml of 5 M KOH solution in a polyethylene flask. A precipitate of red-brown ferrihydrite was obtained, and this suspension was diluted to 2 l with distilled water and held in a closed polyethylene flask at 70 °C for 60 h in the oven. The vessel was removed from the oven and centrifuged, and the precipitate was washed by dialysis, filtered by Millipore glass membrane vacuum filtration system and dried.
Preparation of composite
The composite sample was prepared using modified method of Kosmulski et al. (2003). Iron oxide–activated carbon powder in the ratio 1:5 was dispersed in 0.1 M HNO3 solution and was vigorously stirred at 1800 rpm for 2 h. The mixture was aged for 24 h at 80 °C in the oven. The precipitate was centrifuged and washed to neutrality and then dried in the oven (Kosmulski et al. 2003).
Physicochemical and spectroscopic characterization
Physicochemical parameters such as pH, point of zero charge, bulk density and surface area were used to characterize the prepared sample.
To determine the pH, 1 g of the activated carbon was agitated in a beaker containing 50 ml of deionized water, shaken for 5 min and then allowed to stand for 30 min (Adegoke et al. 2013). The pH was then taken with a pH meter (ATpH model). The bulk density was determined using ASTM D 2866-94. Potentiometric mass titration method was used to determine the point of zero charge (Balderas-Harnandez et al. 2006). The BET surface area analyser was used for surface area determination, and this gives reliable result on the mesopore, micropore and macropore.
Spectroscopic characterization was carried out using scanning electron microscopy (Carl Zeiss ultra plus field emission electron microscope (FESEM) at 5 kV), transmission electron microscopy (JEOL TEM 1010 transmission electron microscope at 200 kV), X-ray diffraction (PW 3050/60 goniometer), X-ray fluorescence (Kevex Fisons Analyst 771), energy-dispersive X-ray spectroscopy (EDX) and Fourier transform infrared spectroscopy (Shimadzu 8400).
Adsorption studies
Preparation of the stock solution of the Cr(VI)
K2Cr2O7 was used in the preparation of the stock solutions. Solution of Cr(VI) 1000 ppm was prepared by weighing 2.826 g of K2Cr2O7 (Sigma-Aldrich), dissolving it in deionized water and making up to 1000 ml mark of the standard flask and adjusted to a pH of 3 as observed from the speciation diagram for chromium. The standard solutions (10–500 ppm) were prepared through serial dilution. The various standard concentrations were determined on a UV–visible spectrophotometer (Shimadzu model) at a wavelength of 354 nm.
Removal of heavy metal ions from aqueous solution
Batch experiments were conducted to investigate the effects of adsorbate concentration, adsorbent dose, contact time, pH and temperature on the adsorption of Cr(VI) on each of the adsorbents (goethite, activated carbon and their composite). All reagents used were of analytical grade. Twenty-five milliliters of the prepared solutions with concentrations 10–500 ppm was measured into a 100-ml conical flask each, and 0.1 g adsorbent was added, agitated using a mechanical shaker for 180 min and then filtered. The initial and final concentrations of Cr(VI) ion were determined on a UV–visible spectrophotometer (Shimadzu model) at a wavelength of 354 nm. Effect of adsorbent dose and contact time was carried out within the range of 0.02–1.0 g and 5–240 min, respectively. Then, the adsorption of Cr(VI) ions under different pHs (1–10) and temperatures (303–334 K) was studied.
The metal concentration adsorbed by the adsorbent phase (Qe, ppm) and % adsorbed were calculated using the following equations (Al-Degs et al. 2006):
where V is the volume of solution (ml), S amount of dry adsorbent/substrate (g), Ci initial metal concentration (ppm), Cf final metal concentration(ppm) and Qe amount absorbed.
Adsorption isotherms, kinetics and thermodynamics
The adsorption capacity data were fitted into the linear forms of some isotherms. These include Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms, and their equations are as follows, respectively:
where
Kinetic models were used to examine the rate of the adsorption process and potential rate controlling step. The experimental data were fitted into the pseudo-first-order, pseudo-second-order, Elovich and intra-particle diffusion kinetics to examine the adsorption kinetics of Cr(VI) in aqueous solution. Thermodynamic parameters such as the free energy (∆G°), standard enthalpy (∆H°) and standard entropy (∆S°) changes during adsorption were evaluated from van’t Hoff plots and calculated by the following equations:
Desorption studies
The desorption experiment study was carried out by contacting 0.02 g of each adsorbent with 25 ml Cr(VI) at concentration range of 10–400 mg/l for 180 min. After the adsorption experiment, the adsorbents were collected by filtration and allowed to dry. Then, they were transferred separately into 25 ml desorbent solutions: 0.1 and 1.0 M HCl and 0.1 and 1.0 M HNO3, agitated for 180 min and filtered. The initial and final concentrations of Cr(VI) ion were all determined on a UV–visible spectrophotometer (Shimadzu model) at a wavelength of 354 nm. The % desorbed desorption efficiency and the desorption index were calculated by the following equations, respectively:
Results and discussion
Physicochemical parameters
The prepared goethite (G) particle is golden yellow which is in line with the result reported in the literature (Schwertmann 1991). The carbon is black in nature, while G–AC composite is greenish-blank in appearance. The percentage yields are 76.65, 54.6 and 94.5 for G, AC and GAC, respectively. This gives the order of GAC > G > AC. GAC gives the highest yield value because little or no proportion was eliminated during its preparation.
The pH is 8.9, 7.2 and 5.5 for G, AC and GAC, respectively. The pH value (8.9) of G shows that it is basic. This alkalinity nature can be associated with the dissociation of OH− present in the compound when dissolved in deionized water due to hydrolysis. The pH value is found to be within ranges of pH obtained for goethite particles elsewhere (Adegoke et al. 2013).
The bulk density of G, AC and GAC was 0.4408, 0.3316 and 0.3638 g/ml, respectively. The higher bulk density of the goethite particle is prone to agglomeration and compactness in nature. A denser precursor or starting material will produce a more compact and harder activated carbon. The precursor used for the AC was found to be dense, so the bulk density of the AC was found in this range and further modification of the AC adsorbent also causes a reduction in the bulk densities. The bulk densities obtained in this study were found to be higher than those reported elsewhere (Naiya et al. 2009) and less than some (Itodo et al. 2009 (0.53 g/m3); Nale et al. 2012 (0.45 g/m3)) where agricultural waste are used as precursors.
The volatile matter obtained for the AC adsorbent is 44.3%, which is higher than the value (20.96%) reported elsewhere (Raffiea et al. 2012). Volatile matter is due to the presence of organic compounds present in the raw material. It was stated that at longer carbonization period, more volatiles are released from the char, thereby resulting in a higher burn off and a corresponding lower yield (Martinez et al. 2006). The moisture content of the activated carbon was obtained at 10.23%, and ash contents for AC were 26.83%, lower than the value reported by Aloko and Adebayo (2008) (29.24%) and higher than those reported elsewhere (Salman et al. 2011 (10.5%); Mane et al. 2013 (5.6%); Elnasri et al. 2013 (5.9%)) where agricultural materials are used.
The iodine number measures the activity level of AC; the higher the number, the higher the degree of activation and the development of the microporous structure (Raffiea et al. 2012). The iodine number of AC prepared was 152.06, and this is relatively lower when compared with the one reported in the literature (Raffiea et al. 2012). Some activated carbons have been reported to be between 600 and 1100 mg/g (Aziz et al. 2009; Baccar et al. 2009).
The PZC is usually the pH value at which a solid immersed in an electrolyte exhibits zero net electrical charge on the surface (Lyklema 1995; Kirby 2010). The pHpzc of G, AC and GAC is 8.8, 7.6 and 7.92, respectively. These are within range as reported in the studies (Adegoke et al. 2013; Elnasri et al. 2013).
Surface area and micropore volume are the key factors in determining whether the material is suitable for the adsorption of metal ion or dyes from aqueous solutions. In addition, the nature of the adsorbent–adsorbate interaction must also be considered. It is understood that the pore volume contributed to the accommodation of adsorbate on the adsorbent (Subbaiah et al. 2011). From this study, the surface area of the sample was determined to be 130.098 m2/g for goethite and this is within the specific surface area range of 8–200 m2/g stated by Cornell and Schwertmann (2003). The micropore volume is 6.3847 × 10−3 cm3/g, and a micropore area is 26.3089 m2/g.
From the physicochemical properties analysis for the three adsorbents, it is observed that the adsorbents possess most of the desirable characteristics of an adsorbent.
Spectroscopic characterization
The X-ray fluorescence analysis result of the goethite revealed that the major oxides found in the samples are Fe2O3, SiO2, CaO, P2O5, In2O3, Ag2O in which iron oxide is the dominant oxide and the minor oxides are MnO, Cr2O3, ZnO, etc. It is shown that 96.97% and 73.18% iron oxide are present in the G and GAC particles, respectively. This shows that the iron oxide is of high purity.
To examine the crystalline structure of the synthesized adsorbents, XRD analysis shows characteristics peaks at angle 2ϴ. Peaks are found at positions of 21.40°, 34.50°, 35.80°, 40.70°, 41.80°, 54.60° and 59.10° for G which are in good agreement with values found elsewhere (Jaiswal et al. 2013; Nassar 2012; Salami and Adekola 2002). The activated carbon spectra show there is low appearance of crystallites on the surface because of its amorphous nature. The spectra of the GAC show peaks 21.60°, 34.50°, 37.10°, 42.80°, 54.50° and 59.40° almost the same spectra with that of the G particle but a little difference because of the slight shift in the peaks caused by the compositing. This is also in agreement with that found in the literature.
The FTIR spectra from Table 1 and also the spectra in Fig. 1a–c reveal that goethite G showed a broad band at 3132.40 cm−1 which is due to the O–H stretching frequency. The stretching frequency of Fe–OH is found at the 863.04 cm−1 and the peak at 769.60 cm−1 is due to the presence of O–H deformation which are diagnostic bands found in goethite. This is in line with the results found elsewhere (Castro et al. 2009; Salami and Adekola 2002). In the spectra of the composite (GAC), the combination of bands found in both goethite and the activated carbon is present with just slight shifts due to the mixing of the goethite particle with activated carbon.
The scanning electron micrograph spectra in Fig. 2a–f show goethite particle that is needle-like in shape and not of uniform size with a diameter of selected rods with values ranging from 97.89 to 182.77 nm. The activated carbon shows a network of pores structure which is not very porous but shallow. The composite micrograph shows needle-like goethite particle embedded on the activated carbon surface.
Adsorption study results
Calibration curve (UV–visible spectroscopy)
The calibration curve for the different concentrations of Cr(VI) is shown in Fig. 3a. The UV–visible spectrum of Cr(VI) in Fig. 3b shows that Cr(VI) has a prominent peak at around 354 nm.
Effect of initial concentration
Investigation of adsorption capacity of Cr(VI) was done on the three adsorbents, namely goethite(G), activated carbon (AC) and their composite (GAC). Three sorption systems, namely Cr//G, Cr//AC and Cr//GAC, were studied at a temperature of 26 ± 2 °C and 0.1 g adsorbent dosage for 180-min contact time. The results obtained for the various adsorption systems are shown in Fig. 4a–c.
The extent to which sorption occurs in a system has been reported to be a function of initial concentration of the adsorbate (metal ions) (Ahalaya et al. 2005). The effect of initial Cr(VI) concentration on the uptake capacity (Q in m/g) for Cr//G, Cr//AC and Cr//GAC sorption systems is shown in Fig. 4a–c, respectively, with an initial concentration of Cr(VI) varying from 10 to 500 mg/l. It can be observed that there was an increase in adsorption capacity Cr(VI) ion onto the adsorbents with an increase in concentration. This shows that the adsorption capacity of Cr(VI) is greatly dependent on the initial concentration. It can be seen that the amount adsorbed in the Cr(VI)//G system at equilibrium increased from 1.499 to 6.630 mg/g, while adsorption capacity of Cr(VI)//AC and Cr(VI)//GAC system also increased from 1.513 to 5.456 and 1.084 to 6.354 mg/g, respectively. The maximum percentage adsorption onto the three adsorbents (goethite, activated carbon and goethite–activated carbon) was found to be 45.585, 46.011 and 32.954, respectively. It can be established that activated carbon adsorbed more compared to goethite and goethite–activated carbon composite. The trend observed can be due to the fact that less favourable sites become involved with increasing metal concentrations in the aqueous solution (Bektas and Kara 2004). Initial concentrations of 50 and 100 mg/l were used for further studies.
Effect of adsorbent dosage
The effect of the adsorbents dosage on the various adsorption systems studied is shown in Fig. 5a–c for the three adsorbents onto Cr(VI) of two concentrations (a = 50 and b = 100 ppm). This gives a total of six systems, namely Cr//aAC, Cr//bAC, Cr//aG, Cr//bG, Cr//aGAC and Cr//bGAC.
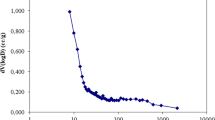
It was observed that in all the systems, the uptake capacity decreased with an increase in adsorbent dose, while the percentage adsorbed steeply increased with adsorbent dose loading between 0.02 and 1 g. Optimum adsorption was observed using 0.02 g of the adsorbent in all systems with corresponding values of 3.736, 5.697, 4.11, 4.489, 5.696 and 6.205 mg/g and percentage adsorbed of 41.07, 31.28, 89.72, 48.30, 100 and 77.82 for various adsorbate–adsorbent, Cr//aAC, Cr//bAC, Cr//aG, Cr//bG, Cr//aGAC and Cr//bGAC systems. Many factors contribute to the optimum adsorbent dosage. One of the factors is sites number available for adsorption. It increased by increasing the adsorbent dose; agglomeration of adsorbent particle at higher concentrations is also another factor. Similar trend was reported in the literature where adsorption capacity of Cr(VI) ions decreases with increasing adsorbent (Dakiky et al. 2002; Archarya et al. 2009; Jaiswal et al. 2013). The increase in adsorbent dosage beyond maximum adsorption capacity of 0.02 g dose resulted in a decline in amount adsorbed which may be attributed to the overlapping of the adsorption sites when the quantity is above 0.02 g. It was observed by some researchers that higher adsorbent dosage could impose a screening effect on the dense other layer of the cells, thereby shielding the binding sites from the metal ions (Pons and Fuste 1993). Adsorbent mass of 0.02 g was used for further studies.
Effect of contact time
The time profile diagram for the adsorption of Cr(VI) on the three adsorbents, namely G, AC and GAC, is represented in Fig. 6a–c. It can be observed that the amount adsorbed increases with an increase in contact time to the peak after which equilibrium is reached at 60 min for goethite (G), 180 min for activated carbon (AC) and 30 min for goethite–activated carbon composite (GAC) with the adsorption capacities (at both 50 and 100 ppm initial concentration) of 24.689 and 6.794 mg/g, 10.04 and 6.905 mg/g and 6.871 and 6.734 mg/g, respectively.
An adsorption time of 60, 180 and 30 min was selected as the equilibrium time for G, AC and GAC, respectively, based on the results of the contact time studied and used for further adsorption studies in investigating other parameters. The effect of contact time on adsorption is important in adsorption processes, because it exercises a great deal of influence on the adsorption capacity (Baysal et al. 2009). It can be observed that the adsorption capacity of the metal ion increased with increasing time. The adsorption process can be divided into two stages, the first initial rapid metal sorption rate which increases to the maximum peak adsorbed and the sharp fall to an equilibrium phase. Initially, there were a large number of vacant active sites available at the initial stages of adsorption and large amount of Cr(IV) ions were bound rapidly on the adsorbents at a faster adsorption rate. The binding site shortly became limited due to repulsive forces between adsorbate ions on the solid and in the solution, and it was difficult for the ions to occupy the remaining surface site. Similar results have been reported elsewhere (Veena Devi et al. 2012).
Effect of pH
The maximum amount adsorbed for the removal of Cr(VI) ion was observed at pH 3. As shown in Fig. 7a–c, adsorption capacity, however, increased as pH was increased from 1.0 to 3.0. Thereafter, the amount decreased from pH 4 to 10 for all adsorbent types: G, AC and GAC (at initial concentrations of 50 and 100 ppm) with values of 6.39 and 49.72 mg/g, 31.46 and 48.69 mg/g and 6.87 and 28.49 mg/g, respectively. pH is an important environmental factor that influences site dissociation (protonation and deprotonation of functional groups on adsorbents). It also affects the solution chemistry of heavy metals, hydrolysis and the speciation of the heavy metals. According to simple speciation diagrams for Cr(VI), all the species occurring at acidic pH values carry a negative charge either as CrO42−, HCrO4− and Cr2O72−.
The decrease in the adsorption efficiency at higher pH can be attributed to an increase in the concentration of OH− ions that hinder the diffusion of Cr(VI) ions. The surface of the adsorbent is positively charged and hence negatively charged Cr2O72− ions which are held by electrostatic forces of attraction. These are in agreement with other literature where the adsorption of Cr(VI) ion is maximum between pH range of 2 and 4 (Veena Devi et al. 2012; Jaiswal et al. 2013).
Effect of temperature
The thermal effect of the various sorption systems and adsorption studies was carried out at temperatures of 303, 313, 323 and 333k.
The temperature profile diagram for the adsorption system of Cr(VI) onto G, AC and GAC is shown in Fig. 8a–c, respectively. There was an increase in adsorption capacity as the temperature was increased in almost all the systems, pointing towards endothermic process. Maximum adsorption capacity was achieved at 333k for G, AC and GAC (at initial concentrations of 50 and 100 ppm) with values 2.34 and 16.75 mg/g, 10.81 and 14.33 mg/g and 8.77 and 15.88 mg/g, respectively. The Cr(VI) ion adsorption is favoured at higher temperature which could be due to the fact that higher temperature activates the metals ions for enhancing adsorption at the coordinating sites of the adsorbents. Furthermore, increasing the temperature will increase the rate of adsorbate diffusion across the external and internal boundary layer because liquid viscosity decreases as temperature increases. It has also been observed that cations move faster with increasing temperature due to the weakness in the electrostatic force of attraction which decreases solvation and make the ions to be smaller (Buasri et al. 2012).
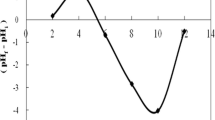
From the temperature profile diagram of both G and GAC adsorption systems, the irregular trend exhibited as temperature was increased which could be attributed to the formation of unstable adsorbate–adsorbent complex.
Adsorption isotherms
The equilibrium experimental data obtained in this study were fitted into four isotherm models, namely Langmuir, Freundlich, Temkin and Dubinin–Radushkevich adsorption isotherm models. Isotherms parameters gotten for the various systems are represented in Table 2.
Langmuir adsorption isotherms
The value of RL indicates the adsorption nature to be either unfavourable if RL > 1), linear if RL = 1, favourable if 0 < RL < 1 and irreversible if RL = 0. From the data obtained from the plot (Table 2), the RLs are greater than 0 but less than 1 for all the three Cr(VI) systems, indicating that Langmuir isotherm is favourable for the three systems. The RLs (the separation factor) are 0.0497, 0.056 and 0.1246, indicating that the equilibrium adsorptions were favourable in the three systems. The maximum monolayer coverage capacity (Qmax) from Langmuir isotherm model was determined to be 4.1304, 4.8450 and 6.4725 mg/g for G, AC and GAC, respectively, KL (Langmuir isotherm constant) 0.0352, 0.0309 and 0.0129 L/mg for G, AC and GAC, respectively, and the R2 values 0.9946, 0.9971 and 0.9877 showing that the sorption data fitted well to Langmuir Isotherm model and the order of their fitness is AC > G > GAC.
The correlation coefficient of 0.9946, 0.9971 and 0.9877 was obtained for Cr(VI) sorption on G, AC and GAC, respectively. This showed that the adsorption systems are monolayer with the highest being Cr(VI)//G system and the least being Cr(VI)//GAC system (Figs. 9, 10).
Freundlich adsorption isotherm
For the Cr(VI) system, the value of 1/n is 0.3144, 0.3181 and 0.4508, while n is equal to 3.181, 3.14 4 and 2.218 for G, AC and GAC, respectively, which lies between 1 < n < 10, indicating that the sorption of Cr(VI) onto the three adsorbents, namely G, AC and GAC (Table 2), is favourable and the 1/n values showed the extent of heterogeneity to be in the order of G > AC > GAC. If value of 1/n < 1, it indicates a normal adsorption, and if 1/n > 1 it indicates cooperative adsorption. Specifically, the linear least-squares method and the linearly transformed equations have been widely applied to correlate sorption data where 1/n is a heterogeneity parameter, the smaller 1/n, the greater the expected heterogeneity. This expression reduces to a linear adsorption isotherm when 1/n = 1. If n lies between 1 and 10, this indicates a favourable sorption process.
The value of Kf was calculated to be 1.4370, 1.3842 and 2.6248 for G, AC and GAC, respectively. This shows that the adsorption capacity of the three adsorbents on Cr(VI) is in the order of GAC > G > AC. The R2 values are 0.8595, 0.9418 and 0.9732 for G, AC and GAC, respectively, which showed that Cr(VI) favours multilayer adsorption on heterogeneous surface of the three adsorbents and the extent is also in the other of GAC > AC > G.
From the correlation coefficient obtained for Cr(VI) sorption (0.8595, 0.9418 and 0.9732) on G, AC and GAC, respectively, it can be deduced that the Cr(VI) Freundlich adsorption systems fitted with Cr(VI)//GAC being the most fitted and Cr(VI)//G being the lowest.
Temkin adsorption isotherm
For the adsorption systems of Cr(VI), the KT values calculated from the plot (Fig. 11) are 2.8468, 2.4334 and 6.4323 and B values of 0.9432, 0.9339 and 1.3356 for Cr(VI)//G, AC and GAC systems, respectively. This shows that KT and B have the highest value in Cr//GAC, which indicates a higher heat of adsorption and lower binding energy. These low values of the binding energies and heat of adsorptions of the three systems indicate physical adsorption. The R2 values (0.7358, 0.9284 and 0.9495 for Cr//G, AC and GAC, respectively) showed that the three systems fitted into the isotherm model with the Cr//GAC being the most fitted and the Cr//G being the least.
Dubinin–Radushkevich isotherm model
From the linear plot of DRK as shown in Fig. 12, the qs value determined is 4.4975, 4.6646 and 5.3495 for Cr//G, AC and GAC, respectively. The mean energy value determined for the Cr(VI) systems is 50, 50 and 40.83 kJ/mol for G, AC and GAC, respectively. The R2 values for the Cr(VI) system (0.8174, 0.9429 and 0.9486 for Cr//G, AC and GAC) showed that the systems fitted well into the model.
Generally, the adsorption of Cr(VI) fitted best into the Langmuir adsorption isotherm than the other three model used as seen in the data analysis above because they have the highest set of R2 values (0.9946, 0.9971 and 0.9877 for Cr(VI)//G, AC and GAC, respectively). The most fitted of all the three systems is Cr(VI)//G system. The order of the isotherm fitness to the systems is Langmuir > Freundlich > DRK > Temkin in the Cr(VI) systems.
Adsorption kinetics
The adsorption kinetics is necessary for the design of adsorption systems, and this explains how fast the rate of chemical reaction occurs and also the factors affect the reaction rate. In order to determine the applicability of the adsorption process in aqueous solution, kinetic studies such as pseudo-first order, pseudo-second order, Elovich and Intra-particle diffusion were investigated for Cr(VI) adsorption system.
Pseudo-first-order kinetics
The linearized pseudo-first-order plots of the adsorption of Cr(VI) in two different initial concentrations (50 and 100 ppm) onto the three adsorbents: goethite (G), activated carbon (AC) and goethite–activated carbon composite (GAC), are shown in Fig. 13. The correlation regression coefficient R2 of the adsorption system as seen in the pseudo-first-order plot shows a poor fitting since most of the values are lower than 0.5 except that of the Cr//AC(100) adsorption system which has a good fitting (R2 = 0.9356). It can be observed from the pseudo-first-order plot that the adsorption of Cr(VI) metal (with initial concentrations of 50 and 100 ppm) onto the three forms of adsorbents, that Qe calculated were by far lower than the experimental Qe. This also shows that the pseudo-first-order model fitted less.
Pseudo-second-order kinetics
The plot obtained for the pseudo-second-order model represented the experimental data better compared to the pseudo-first-order kinetic model as shown in Fig. 14. The pseudo-second-order kinetic plots are of better linearity with a correlation coefficient not less than 0.97 for the metal at all concentrations except for Cr//AC system whose value is 0.9023 (Fig. 15).
The comparisons between experimental and theoretically calculated Qe values at all concentrations for the Cr(VI) adsorption onto the three forms of adsorbents as shown in Table 3 show a better agreement than in the pseudo-first-order model. This showed that the pseudo-second-order kinetics fitted better than the pseudo-first-order kinetics for the adsorption of Cr(VI) in aqueous solution by G, AC and G–AC.
Elovich kinetic model
The Elovich kinetic model has been reported to be one of the most useful models for describing chemisorption on highly heterogeneous adsorbent.
The low correlation coefficient values obtained from the plots of the Elovich equation as shown in Table 3 showed that the adsorption systems are in poor agreement with this kinetic model since the R2 < 0.5 except for the Cr//G(100 ppm) and Cr//AC(100 ppm) systems which have their R2 > 0.5.
Intra-particle diffusion model
This kinetic model describes rate of diffusion in terms of external diffusion which might take place during the adsorption process (Allen et al. 1989). The intra-particle diffusion plots are shown in Fig. 16. The data obtained from the plot of this kinetic model gave correlation coefficients that were very low (R2 < 0.5) except for the Cr//AC(100 ppm) and Cr//GAC(100 ppm) with the R2 values 0.928 and 0.509, respectively, as shown in Table 3. This implies that the experimental data did not fit properly with intra-particle diffusion equation.
The pseudo-second-order kinetic model best fitted into the adsorption systems with good correlation coefficients values (R2 > 0.9), while the other kinetic models used (pseudo-first order, Elovich and intra-particle diffusivity) showed poor agreement in most of the adsorption systems.
Thermodynamics studies
Thermodynamic parameters can be useful in evaluation of orientation of the physicochemical adsorption reaction and feasibility of the reaction as well as the stability of the adsorbed phase. The data derived from the effect of temperature study were used for feasibility of the adsorption process of Cr(VI) ion onto goethite (G), activated carbon (AC) and G–AC composite. The thermodynamic parameters such as the change in enthalpy, entropy change and the change in the Gibb’s free energy of the adsorption process for the various systems were determined using the appropriate equations. The result obtained from the thermodynamic study is shown in Table 4.
The correlation coefficient R2 obtained for the thermodynamic studies is shown in the table above. The systems give good correlation values except for Cr//GAC(100) system that gives a low value (R2 < 0.5). These relatively low values (compared to others) can be attributed to the irregular trend that exhibited in its respective temperature profile diagram. From Table 4, it can be observed that the values for ∆G are all positive. The Gibb’s free energy values indicate the degree of spontaneity of adsorption process. This implies a non-feasible and non-spontaneous reaction system over the temperature range studied.
The enthalpy change ∆H value signifies whether an adsorption process is endothermic or exothermic in nature. In some of the systems such as Cr//G(50), Cr//G(100) and Cr//GAC(100), ∆H is found to be negative, which indicates that the reaction that occurs in these systems is exothermic, while those of systems Cr//AC(50 and 100) and Cr//GAC(50) are endothermic in nature because of their positive ∆H values and possible strong bond between the metal ion and the adsorbents (Aksu and Tune 2005). The low value of ∆H suggests that the adsorption process is likely to be physisorption (Hema and Arivoli 2007).
The entropy change ∆S is a measure of the degree of disorderliness and randomness of a system. From the table, the ∆S value for systems Cr//G(50), Cr//G(100), Cr//AC(100) and Cr//GAC(100) is negative, and this indicates a decrease in the disorderliness and randomness at the adsorbent–adsorbate interface and affinity for adsorbent material, while the ∆S value for systems Cr//AC(50) and Cr//GAC(50) is positive, indicating increased disorderliness and randomness at the adsorbent–adsorbate interface (Lawal et al. 2010; Rajashree et al. 2012).
Desorption studies
Desorption studies is important in the adsorption process because it provides the re-usability and the economic importance of an adsorbent. The results in Table 5 show the % desorption of Cr(VI) by 0.1 M and 1.0 M of HNO3 and 0.1 M and 1.0 M HCl. The maximum percentage desorbed within 2 h was found to be 94.60 for Cr//G, 91.81 for Cr//AC and 36.33 for Cr//GAC in 1.0 M HNO3. The result in the tables reveals that % chromate desorbed was higher when HNO3 was used and lower when HCl was used. It can be seen that the use of HNO3 as a desorbing agent in the studies gives a higher desorption efficiency, and this increases as the concentration of the acid increases. The relatively higher value recorded for HNO3 over HCl could be due to its complexing ability. When HNO3 is used as desorbing agent, the adsorbent surface was covered with H+ ions, while the coordination sphere of the Cr(VI) ions was disrupted and then metal ions were released into the solution. This is possible because H+ in the solution replaces the already embedded metal ion on surface of the adsorbents as they were dissolved into the acidic solution. It has been reported that metal ions could not compete with H+ ions for exchange sites and subsequently heavy metal ions were released into the solution. The FTIR results also complement this result. The bonds of chromate-loaded iron oxides were shifted, and the intensities of the bands were higher (Fig. 17).
The desorption index (DI) of G, AC and GAC for Cr(VI) is also shown in Table 5. A sorption process is considered to be completely irreversible when DI equals 1. The degree of irreversibility of a sorption process increases as DI value deviates from 1 (Adegoke et al. 2014). In this study, the DI values for all the systems are all less than 1, which implies that the sorption process is practically reversible at all concentrations studied except for G//Cr and AC//Cr with the DI values of 2.566 and 3.689 when 1.0 M HNO3 was used as the desorbing agent.
Conclusion
Three adsorbents, namely goethite, activated carbon and goethite–activated carbon, have prepared in the laboratory. The batch adsorption shows that the three adsorbents were effective for the removal of Cr(VI) ions from aqueous solutions. It was observed that the effectiveness of the adsorbents increases in the other GAC < AC < G. The adsorption experiments were carried out as a function of concentration, contact time, adsorbent dose, pH and temperature. Initial concentration of the metal ion was found to greatly affect adsorption process, and acidic conditions and moderate temperatures were shown to favour the adsorption process. Equilibrium was reached at 60, 120 and 90 min for G, AC and GAC, respectively, and adsorbent dose was 0.02 g.
The isotherms study showed that the equilibrium data of Cr(VI) ions on G, AC and GAC fitted best into the Langmuir isotherm model than the others indicated by the correlation coefficient values obtained from the equilibrium graphs. The adsorption kinetic data were best explained by the pseudo-second-order kinetic model implying chemisorption process as the rate limiting step. From the thermodynamic analysis, the values of ∆G for all the systems in the adsorption of Cr(VI) onto the three adsorbents were found to be positive, which indicates a non-spontaneous system. Some systems (Cr//G(100) and Cr//GAC(100)) are exothermic, that is, ∆H is negative, while others are positive indicating an endothermic system. The positive values of ∆S indicate that there is an increase in the disorderliness in solid/solution interface during the adsorption processes. Desorption studies revealed that 1.0 HNO3 was better desorbing agent than 0.1 HNO3, 0.1 HCl and 1.0 HCl, and Cr(VI) was well desorbed.
The result of this our study indicated that goethite–activated carbon composite can be used for the removal of Cr(VI) ions from aqueous solution.
References
Adegoke HI, Adekola FA, Fatoki OS, Ximba BJ (2013) Sorption interaction of oxyanions with iron oxides; a review. Pol J Environ Stud 22(1):7
Adegoke HI, Adekola FA, Fatoki OS, Ximba BJ (2014) Adsorption of Cr(VI) on synthetic Hematite (α-Fe2O3) nanoparticles of different morphologies. Korean J Chem Eng 2(1):142–154
Ahalaya N, Kanamadi RD, Ramanchandra TV (2005) Biosorption of chromium(VI) from aqueous solution by the husk of Bengal gram (Cier arientinum). Electron J Biotechnol 8:71–78
Aksu Z, Tune O (2005) Application of biosorption for penicillin G removal comparison with activated carbon. Process Biochem 40:831–847
Al-Degs S, El-barhouthi MI, Issa AA, Khaisheh MA, Walker GM (2006) Sorption of Zn(II), Pb(II) and Co(II) using natural sorbents: equilibrium and Kinetic studies. Water Res 40:2645
Allen SJ, McKay G, Khadar KYH (1989) Kinetics and equilibrium study of chromium adsorption on zeolite. Environ Pollut 56:39–50
Aloko DF, Adebayo GA (2008) Production and characterization of activated carbons from agricultural waste. J Eng Appl Sci 2(2):440–444
Ambashta R, Sillanpaa M (2010) Water purification using magnetic assistance; a review. J Hazard Mater 180(1–3):38–49
Archarya J, Sahu JN, Mohanty CR, Meikap CB (2009) Removal of lead (II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem Eng J 14:249–252
Asghar A, Aziz A, Raman A Mohd, Wan A (2015) Advanced oxidation processes for in situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J Clean Prod 87:826–838
Aziz A, Ovali MS, Elanduloussi EH (2009) Chemically modified olive stone a low coat sorbent for heavy metals and basic dyes removal from aqueous solution. J Hazard Mater 163:441–447
Baccar R, Bouzid J, Feki M, Mountel A (2009) Preparation of activated carbon from Tunisian olive waste cakes and its application for adsorption of heavy metal ions. J Hazard Mater 162:1522–1529
Balderas-Harnandez P, Ibanez JG, Godinez-Raminez JJ, Almada-Calvo F (2006) Microscale environment chemistry: part 7, estimation of point of zero charge for simple metal oxides by a simplified potentiometric-mass titration method. Chem Educ 11:267–270
Baysal Z, Cinar E, Bulut Y, Alkan H, Dogu M (2009) Equilibrium and thermodynamic studies on biosorption of Pb(II) onto Candida albican biomass. J Hazard Mater 161:62–67
Bektas N, Kara S (2004) Removal of lead from aqueous solution by natural clinoptilolite equilibrium and kinetics studies. Sep Purif Technol 39(3):189–200
Buasri A, Chaiyut N, Tapang K, Jaroensin S, Panphrom S (2012) Biosorption of heavy metals from aqueous solution using water hyacinth as a low cost biosorbent. Civ Environ Res 2(2):17–25
Cornell RM, Schwertmann U (2003) The Iron oxides structure, properties, reactions, occurences and Uses. VCH, Weinheim, pp 395–432
Dakiky M, Khamis M, Manassra A, Mereb M (2002) Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv Environ Res 6:533–540
Dizadji N, Abootalebi AN (2011) Adsorption of Cr and Cu in aqueous solution using tea residue. Int J Environ Sci Technol 8(3):631–638
Elnasri NA, Mutaz AE, Mohammed AE (2013) Physico-chemical characterization and Freundlich isotherms of adsorption of Fe(II) from aqueous solution by using activated carbon prepared from Doum fruit waste. Arch Appl Sci Res 5(5):149–158
Faraji M, Yamini Y, Rezaee M (2010) Magnetic nanoparticles; synthesis, stabilization, functionalization, characterization. J Iran Chem Soc 7(1):1–37
Franca RDG, Vieira A, Mata AMT, Carvalho GS, Pinheiro HM, Lourenco ND (2015) Effect of an azo dye on the performance of an aerobic granular sludge sequencing batch reactor treating a simulated textile wastewater. Water Res 85:327–336
Hema M, Arivoli S (2007) Comparative study on the adsorption kinetics and thermodynamics of dyes onto acid activated low cost carbon. Int J Phys Sci 2(1):10–17
Itodo AU, Abdulrahman FW, Hassan LG, Maigandi SA, Happiness UO (2009) Thermodynamic equilibrium, kinetics and adsorption mechanism of industrial dye removal by chemically modified poultry droppings activated carbon. Niger J Basic Appl Sci 17(1):38–48
Jain M, Garg VK, Kadirvelu K (2009) Chromium (VI) removal from aqueous system using Helianthus annus (sunflower) stem waste. J Hazard Mater 162:365–372
Jaiswal A, Banerjee S, Mani R, Chattopadhyaya MC (2013) Synthesis, characterization and application of goethite mineral as an adsorbent. J Environ Chem Eng 1:281–289
Kirby BJ (2010) Micro and nanoscale fluid mechanics: transport in microfluidic devices 3(2):76–77
Kosmulski M, Maczka E, Jartych E, Rosenholm JB (2003) Synthesis and characterization of goethite and goethite–hematite composite. Experimental study and literature survey. Adv Colloid Interface Sci 103:57–76
Lawal OS, Sanni AR, Ajayi IA, Rabiu OO (2010) Equilibrium, thermodynamic and kinetic studies for the biosorption of aqueous lead(II) ions onto the seed husk of Calophyllum inophyllum. J Hazard Mater 177(3):829–835
Lyklema JM (2002) Fundamentals of interface and colloid science. Studi Interface Sci 15:17–73
Malakootan M, Nouri J, Hossaini H (2009) Removal of heavy metals from paint industry’s water using Leca as an available adsorbent. Int J Environ Sci Technol 6(2):183–190
Mane SM, Vanjara AK, Sawant MR (2013) Removal of phenol from wastewater using date seed carbon. J Chin Chem Soc 52:1117–1122
Martinez ML, Tores MM, Guzman CA, Maesri DM (2006) Preparation and characterization of activated carbon from olive stones and walnut shells. Ind Crops Prod 23(1):23–28
Naiya TK, Bhattacharya AK, Mandel S, Das SK (2009) The sorption of lead(II) ions on rice husk ash. J Hazard Mater 163:1254–1264
Nale BY, Kagbu JA, Uzairu A, Nwakwere ET, Saidu S, Musa H (2012) Kinetic and equilibrium studies of the adsorption of Lead(II) and Nickel(II) ions from aqueous solutions on activated carbon prepared from maize cob. Adv Appl Sci Res 3(2):302–312
Nassar NN (2012) Kinetics, equilibrium and thermodynamic studies on the adsorptive removal of nickel, cadmium and cobalt from wastewater by superparamagnetic iron oxide nanoadsorbents. Can J Chem Eng 90(5):1231–1238
Pons MP, Fuste CM (1993) Uranium uptake by immobilized cells of pseudomonas strain EPS 5028. Appl Microbiol Biotechnol 39:661–665
Punzi M, Nilsson F, Anbalagan A, Svensson B, Jonsson K, Mattiasson B, Jonstrup M (2015) Combined anaerobic-ozonation process for treatment of textile wastewater: removal of acute toxicity and mutagenicity. J Hazard Mater 292:52–60
Raffiea BJ, Palanisamy PN, Sivakumar P (2012) Preparation and characterization of activated carbon from Thevetia peruviana for the removal of dyes from textile waste water. Adv Appl Sci Res 3(1):377–383
Rajashree K, Gupta N, Atul Kumar K, Chattopadhyaya MC (2012) Determination of equilibrium, kinetics and thermodynamic parameters of brilliant green dye from aqueous solution onto eggshell powder. Indian J Chem Technol 19:26–31
Salami N, Adekola FA (2002) A study of sorption of cadmium by goethite in aqueous solution. Bull Chem Soc Ethiop 16:1–7
Salman M, Athar M, Shafique U, Din MI, Rehman R, Akram A, Ali SZ (2011) Adsorption modelling of alizarin yellow on untreated and treated charcoal. Turk J Eng Environ Sci 35:209–216
Schwertmann U, Cornell RM (1991) Iron oxide in the laboratory. Weinheim VCH Verlag
Shen YF, Tang J, Nie ZH, Wang YD, Ren Y, Zou L (2009) Preparation and application of magnetic Fe3O4 nanoparticle for wastewater purification. Sep Purif Technol 68:312–319
Subbaiah MV, Yuvaraja G, Vijaya Y, Abburi K (2011) Equilibrium, kinetic and thermodynamic studies on biosorption of Cu(II), Cd(II), Pb(II) and Ni(II) from aqueous solution by Chitosanabrus precatorius blended beads. J Chem Pharm Res 3(2):365–378
Veena Devi B, Jahagirdar AA, Zulfiqar Ahmed MN (2012) Adsorption of chromium(VI) on activated carbon prepared from coconut shell. Int J Eng Res Appl 2(5):364–370
Xu P, Ming Zeng G, Huang DL, Feng CL, Hu S, Hua Zhao M, Lai C, Wei Z, Huang C, Xin XIe G, Feg Liu Z (2012) Uses of iron oxide nanomaterials in wastewater treatment, a review. Sci Total Environ 424:1–10
Zouboulis AI, Lazaridis NK, Karapantsios ThD, Matis KA (2009) Heavy metals removal from industrial waste water by biosorption. Int J Environ Pollut
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical standards
The design of the experiment gives proper respect to the environment by minimizing harm. All chemical wastes were properly and safely disposed after conducting the research work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adebayo, G.B., Adegoke, H.I. & Fauzeeyat, S. Adsorption of Cr(VI) ions onto goethite, activated carbon and their composite: kinetic and thermodynamic studies. Appl Water Sci 10, 213 (2020). https://doi.org/10.1007/s13201-020-01295-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01295-z