Abstract
Research evidence has shown that pollution of surface and underground waters is the leading sources of environmental and health-related problems. Disposed unused therapeutic drugs have been known to contaminate underground water and also offer drug resistance to infection-causing bacterial. This research seeks to evaluate the use of US/PS/Fe3O4 for the removal of ciprofloxacin (CIP-F) from aqueous solutions. The research also seeks to obtain the optimum set of conditions about which the highest removal efficiency of CIP-F is obtained by monitoring the used pH, Fe3O4 nanoparticles (NPs) concentration, PS concentration, CIP-F concentration, and contact time. The analysis was done using a UV–Vis spectrophotometer (Cecil model CE102) set at 280 nm. The result shows that a 98.43% removal efficiency is achievable after optimization if the separation parameters were set to the optimum conditions (pH = 5, CIP-F concentration = 200 mg/L, PS concentration = 0.15 mol/L, Fe3O4 concentration = 0.01 g/L and contact time = 45 min). The reaction was also observed to follow the pseudo-first-order reaction model. Since the results obtained show that US/PS/Fe3O4 can effectively and efficiently aid the surface adsorption of CIP-F from aqueous solutions, it is therefore recommended based on experimental findings that US/PS/Fe3O4 be used for removing CIP-F from effluents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research evidence has shown that pollution of surface and underground water is leading sources of environmental and health-related problems. Continuous usage of antibiotics to mitigate the resulting health issues could result in drug resistance even in low concentrations because they provided resistance to the causative bacteria (Choi et al. 2008). Drugs are generally used for treatment and prevention of bacterial infections (Ahmadi et al. 2017) and, as such, may either kill or inhibit the growth of the bacteria (Choi et al. 2008). Unused therapeutic drugs are sometimes disposed of into the sewage system. If these drugs are not degraded during sewage treatment, in soil or other environmental compartments, they may reach the surface water and groundwater and, potentially contaminate drinking water (Ahmadi et al. 2017). One of such antibiotics is ciprofloxacin (CIP-F). Ciprofloxacin (CIP-F) is a common antibiotic of fluoroquinolone class (Onyechi and Igwegbe 2018) that is widely prescribed for the treatment of intra-abdominal infections; this type of infection is associated with diarrhea, respiratory tract infections, and urinary tract infection (Zhang et al. 2011; Igwegbe et al. 2020). The antibiotic is classified into the fluoroquinolone class, which has a stable naphthol ring and is toxic to microorganisms, and their stability in the environment (Martinez 2009).
Many treatment methods have been proposed for the removal of CIP-F from contaminated waters, which include photodecomposition (Don et al. 2010), adsorption (Khoshnamvand et al. 2017; Rahdar et al. 2019; Dhiman and Sharma 2018), oxidation (Bader and Hoigné 1981), and flotation (Mostafapour et al. 2017). The advanced oxidation process (AOP) is one of the most effective methods of decomposing and removing dangerous, resistive, and biological irresolvable organic pollutants (Ren et al. 2010). This process is based on the production of hydroxyl radicals (Arsene et al. 2011; Poulopoulos et al. 2006; Khan et al. 2019b; Balarak et al. 2019). These radicals oxidize organic compounds swiftly and non-selectively (Arsene et al. 2011). The use of ultrasonic waves has also attracted attention owing to their cheapness, environmentally friendliness, and lack of sludge production. The ultrasonic process is based on the development of pore or micro-bubbles from acoustic cavitation in water (Nelson et al. 2002).
However, the use of advanced oxidation cycle processes alone has not been efficient, its efficiency can be increased by using a sonochemical oxidation process, which is achieved by the addition of chemicals such as Persulfate, nanoparticles, and catalytic particles (Chen and Su 2012). Persulfate oxidation with a redox potential of 2.01 has proven to be more effective when compared with hydrogen peroxide and ozone since the produced radicals are more cost-efficient, soluble, and stable when applied to toxic and resistive materials (Epold et al. 2015). Persulfate (PS) salt dissolved in water will be produced \( {\text{S}}_{2} {\text{O}}_{8}^{2} \) anion with low oxidation capacity. Chemical and thermal methods can be used to increase the oxidative capacity and production of \( {\text{SO}}_{4} \) from the aforementioned anion (Chen and Su 2012). The rate of persulfate reaction has been observed to increased significantly when heat, light, or specific metallic ions were used as a catalyst (Epold et al. 2015). Ions from cobalt or Iron are commonly used metallic ions for the activation of Persulfate (Taimoory et al. 2017). Metallic ions from Iron \( ({\text{Fe}}^{2 + } ) \) are the most preferred but are associated with problems such as substantial volume requirements and high sludge production. To curb this excess, iron oxides nanoparticles can be used as a perfect substitute for \( {\text{Fe}}^{2 + } \) as they are non-toxic, readily available, and cost-effective. Iron oxide nanoparticle produced by green synthesis has been used to replace \( {\text{Fe}}^{2 + } \) in this study to investigate its effectiveness.
This paper presents the removal of (CIP-F) from aqueous solution by the application of US/PS/Fe3O4. During this study, process parameters like pH, persulfate (PS) concentration, Fe3O4 nanoparticles concentration, and time were varied and optimized, to access the optimal removal during the batch studies. The final concentration of CIP-F was investigated using the UV–Vis spectrophotometer Cecil model CE1021 at 280 nm.
Materials and methods
Materials
Ciprofloxacin (CIP-F) with 100% purity was used for this research; the CIP-F used was supplied by Sigma–Aldrich. CIP-F with the chemical formulae: C16H17KN2O4S has a molecular weight of 385.8 g/mol. Figure 1 shows the molecular structure of CIP-F.
Pilot ultrasonic
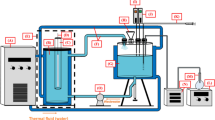
A reactor with a known surface area is used for the pilot test. The reactor is a digital ultrasonic apparatus (Elma CD-Germany, 4820) made from Plexiglas. It has 3.7 L capacity in volume with a 2.5 w/cm2 per unit of energy input and a power input of 500 w per 100 ml samples in the bath with US waves.
Synthesis of Iron (II) sulfate heptahydrate (Fe3O4)
Analytical grade Iron (II) sulfate heptahydrate (FeSO4.7H2O) and sodium hydroxide used in this research were also supplied by Sigma-Aldrich. Also, the magnetite nanoparticles used in this research were synthesized based on earlier published research on the use of a green surfactant-free electrochemical method (Taimoory et al. 2017).
Experimental methods
The pH of the water sample used for the research was adjusted by adding 0.1 mol of HCl into each flask. The final pH of the water sample was measured using a MIT65 pH meter. A stock solution of CIP-F was prepared with a concentration of 1000 ppm in double-distilled water. The prepared CIP-F solution was then poured into the Erlenmeyer flask, and Fe3O4–NPs were subsequently added. The Erlenmeyer flask with its contents was then placed inside the ultrasonic apparatus with the ultrasound waves regulated to 60 kHz at a set time. After the reaction, the residual concentrations were measured using a UV-visible spectrophotometer (Shimadzu Model: CE-1021-UK). The readings were taken at 280 nm with the spectrophotometer. The removal efficiency (%\( R \)) was then calculated using the equation below (Ahmadi and Mostafapour 2017a, b, c):
where \( C_{o} \) and \( C_{f} \) are the initial and equilibrium liquid-phase concentration of CIP-F(mg/L), respectively.
The four process variables monitored in this study were the initial pH of the solution (varied between 3 and 8), the concentration of the nanoparticles (varied between 0.01 and 0.08 g/L), the contact time (varied between 10 and 120 min), the concentration of PS (varied between 0.01 and 1.2 mol/L), and the initial concentration of CIP-F (varied between 50 and 250 mg/L).
Result and discussion
Effect of pH
The effect of pH is one of the parameters being monitored in this research. It directly influences the amount and type of radicals used for the advanced oxidation process. Figure 2 presents the results of varying the pH of 100 mg/L of ciprofloxacin (CIP-F) from 3 to 8 while monitoring its removal efficiency on US/PS/Fe3O4. The results showed that CIP-F removal efficiency increases with increasing pH. The removal efficiency showed a decline after pH 5 was exceeded. The highest possible efficiency was observed at pH 5, showing that CIP-F is better removed in an acidic environment. Also, since the advanced oxidation process works on the principle of hydroxyl production, removing it in an acidic medium encourages a neutralization reaction, thus enhancing the decomposition of the pollutant (Shemer et al. 2006).
Effect of Fe3O4–NPs concentration
Figure 3 presents the result obtained from monitoring the effect of change in concentration of Fe3O4–NPs on the removal efficiency, the optimum conditions were obtained at pH of 5 and Fe3O4–NPs concentration of 0.01 g/L. Increasing the Fe3O4–NPs concentrations from 0.01 to 0.08 g/L had a decreasing effect on the removal efficiency; the increased production of kernel resulted from the increase in Fe3O4–NPs concentration, thereby reducing the number of bubbles and radicals produced, i.e., nanoparticles provide extra surfaces for cavitation (Cheng et al. 2013).
Effects of PS concentration
Figure 4 shows the effect of varying the concentration of persulfate on CIP-F removal efficiency. This was achieved by maintaining the pH at 5. The result shows that the removal efficiency increased when the PS concentration increased from 0.01 to 0.15 mol/L but subsequently reduces with further increase in PS concentration. The reaction mechanism is as shown by Eqs. 2 to 5. PS is converted to sulfate radical (one of the strongest oxidative agents), and it decomposes the organic compounds to water, \( {\text{CO}}_{2 } \) and mineral acids. Also, the produced persulfate radical reacts with water and hydroxyl ions indirectly to produce hydroxyl radical which decomposes organic materials. (Ta et al. 2006; Shiying et al. 2009).
Given the preceding, increasing the concentration of the PS used makes the radicals act as a scavenger and converted persulfate radical to sulfate (Eq. 6) (Li et al. 2011).
Effect of time and CIP-F concentration
This was used to monitor the time effect of varying the concentration of CIP-F concentration on the removal efficiency of CIP-F. The CIP-F concentrations used were 50, 200, and 250 mg/L for a varied duration between 10 and 120 min. The pH and PS concentrations used were set at 5 and 0.15 mol/L, respectively. The results obtained are presented in Fig. 5. Based on the concentration of CIP-F used, the removal efficiency decreased with an increase in concentration, as observed in Fig. 5. The removal efficiency initially increased as the concentration of CIP-F used increased from 50 to 200 mg/L but subsequently reduces as the concentration of CIP-F used was increased to 250 mg/L. This subsequent decrease in the removal efficiency associated with an increase in CIP-F concentration may be attributed to increased production of by-products during the reaction (Asl et al. 2015). It is, therefore, safe to infer that the removal efficiency decreased with an increase in the concentration of US/PS/Fe3O4.
Considering the time effect per concentration, an initial increase in removal efficiency was observed for 45 min, after which a gradual decline began to emerge. It was observed from the research that the optimum removal efficiency was obtained at 45 min. Although a similar study by Khan et al. (2019a) indicated that the optimal resident time was 30 min, their study investigated the catalytic ozonation process for the removal of ciprofloxacin with modified active carbon with MgO in aqueous solution using a fluidized bed reactor (Guo et al. 2014).
Kinetics study
The kinetic study was carried out to investigate the rate of disintegration of CIP-F using the US/PS/Fe3O4 process and to classify the reaction into the appropriate order. Organic chemical compound reactions are either classified into the pseudo-first-order or pseudo-second-order categories and, as such, the research tests to see which category the chemical reaction falls into. The equations for the pseudo-first-order and pseudo-second-order reactions are given below as Eqs. 7 and 8, respectively (Li et al. 2011; Ahmadi et al. 2019; Hemmati et al. 2017):
where Co = Initial concentration in milligrams per liter and C = Final concentration in milligrams per liter at time t.
The test variables were set to the optimum condition (contact time = 10 min, pH = 7, PS concentration = 0.02 mol/L and nanoparticles concentration = 0.25 g/L) for perfect modeling of the rate of reaction. The result obtained for the rate of disintegration and the calculated results from Eqs. 7 and 8 are presented in Fig. 6 and Table 1. From the result, it can be observed that the rate constant obtained is similar to the value obtained from the slope of the plot of \( { \ln }\left( {\frac{c}{{c_{0} }}} \right) \) against the time. It is, therefore, safe to conclude that the reaction follows the pseudo-first-order scheme. The associated loss of CIP-F in the reaction was observed to be a function of time and correctly models the pseudo-second-order scheme (Asl et al. 2015).
Conclusion
The results obtained from this research have shown that the US/PS/Fe3O4 process can effectively and efficiently aid the surface adsorption of CIP-F from aqueous solutions with a 98.43% removal efficiency. This was achieved by carrying out the degradation at the optimum conditions set to pH = 5, CIP-F concentration= 200 mg/L, PS concentration = 0.15 mol/L and Fe3O4 concentration = 0.01 g/L at a resident time of 45 min. The reaction was also observed to follow the pseudo-first-order reaction model rather than the pseudo-second-order reaction. It is therefore recommended based on experimental findings that US/PS/Fe3O4 be used for the removal of CIP-F from effluents.
References
Ahmadi S, Mostafapour FK (2017a) Adsorptive removal of aniline from aqueous solutions by Pistacia atlantica (Baneh) shells: isotherm and kinetic studies. J Sci Technol Environ Inform 5(1):327–335. https://doi.org/10.18801/jstei.050117.35
Ahmadi S, Mostafapour FK (2017b) Tea waste as a low-cost adsorbent for the removal of COD from landfill leachate: kinetic Study. J Sci Eng 4(6):103–108
Ahmadi S, Mostafapour FK (2017c) Survey of efficiency of dissolved air flotation in removal penicillin G potassium from aqueous solutions. Br J Pharm Res 15:1–11. https://doi.org/10.9734/BJPR/2017/31180
Ahmadi S, Banach A, Mostafapour FK, Balarak D (2017) Study survey of cupric oxide nanoparticles in removal efficiency of ciprofloxacin antibiotic from aqueous solution: adsorption isotherm study. Desal Water Treat 89:297–303. https://doi.org/10.5004/dwt.2017.21362
Ahmadi S, Igwegbe CA, Rahdar S (2019) The application of thermally activated persulfate for degradation of Acid Blue 92 in aqueous solution. Int J Ind Chem 10(3):249–260. https://doi.org/10.1007/s40090-019-0188-1
Arsene D, Musteret CP, Catrinescu C, Apopei P, Barjoveanu G, Teodosiuc C (2011) Combined oxidation and ultrafiltration processes for the removal of priority organic pollutants from wastewaters. Environ Eng Manag J 10(12):67–76. https://doi.org/10.30638/eemj.2011.261
Asl FB, Kermani M, Farzadkia M, Esrafili A, Arian S, Zeynalzadeh D (2015) Removal of metronidazole from aqueous solution using ozonation process. J Mazandaran Univ Med Sci 25(121):131–140
Bader HJ, Hoigné J (1981) Determination of ozone in water by the indigo method: a submitted standard method. Water Res 15:449–456. https://doi.org/10.1080/01919518208550955
Balarak D, Igwegbe CA, Onyechi PC (2019) Photocatalytic degradation of metronidazole using BIOI-MWCNT composites: synthesis, characterization, and operational parameters. Sigma J Eng Nat Sci 37:1231–1245
Chen WS, Su YC (2012) Removal of dinitrotoluenes in wastewater by sono-activated persulfate. Ultrason Sonochem 19:921–927. https://doi.org/10.1016/j.ultsonch.2011.12.012
Cheng W, Yang M, Xie Y, Liang B, Fang Z, Tsang EP (2013) Enhancement of mineralization of metronidazole by the electro-Fenton process with a Ce/SnO2–Sb coated titanium anode. Chem Eng J 220:214–220. https://doi.org/10.1016/j.cej.2013.01.055
Choi KJ, Kim SG, Kim SH (2008) Removal of antibiotics by coagulation and granular activated carbon filtration. J Hazard Mater 151(1):38–43. https://doi.org/10.1016/j.jhazmat.2007.05.059
Dhiman N, Sharma N (2018) Batch adsorption studies on the removal of ciprofloxacin hydrochloride from aqueous solution using ZnO nanoparticles and groundnut (Arachis hypogaea) shell powder: a comparison. Indian Chem Eng. https://doi.org/10.1080/00194506.2018.1424044
Don L, Yu T, Zhang Y, Li Z, Lui M, Qi R (2010) Antibiotic resistance characteristic of environmental bacteria from an oxytetracycline production wastewater treatment plant and the receiving river. Appl Environ Microbiol 76(11):3444–3451
Epold I, Trapido M, Dulova N (2015) Degradation of levofloxacin in aqueous solutions by Fenton, ferrous ion activated persulfate and combined Fenton/persulfate systems. Chem Eng J 279:452–462. https://doi.org/10.1016/j.cej.2015.05.054
Guo Y, Zhou J, Lou X, Liu R, Xiao D, Fang C (2014) Enhanced degradation of tetrabromobisphenol A in water by a UV/base/persulfate system: kinetics and intermediates. Chem Eng J 254:538–544. https://doi.org/10.1016/j.cej.2014.05.143
Hemmati H, Bazrafshan E, Kamani H, Mosafer J, Balarak D, Kord Mostafapour F (2017) Optimization of sono-nanocatalytic process using γ-Fe2O3 for penicilin antibiotic removal by response surface methodology. J Torbat Heydariyeh Univ Med Sci 5:1–6
Igwegbe CA, Ahmadi S, Rahdar S, Ramazani A, Mollazehi AR (2020) Efficiency comparison of advanced oxidation processes for ciprofloxacin removal from aqueous solutions: chemical, -nano-chemical and -nano-chemical/persulfate processe. Environ Eng Res 25:178–185
Khan NA, Khan SU, Ahmed S (2019a) Recent trends in disposal and treatment technologies of emerging-pollutants: a critical review. Trends Anal Chem 122:115744. https://doi.org/10.1016/j.trac.2019.115744
Khan NA, Khan SU, Islam DT (2019b) Performance evaluation of column-SBR in paper and pulp wastewater treatment: optimization and bio-kinetics. Desalin Water Treatment 156:204–219. https://doi.org/10.5004/dwt.2019.23775
Khoshnamvand N, Ahmadi S, Mostafapour FK (2017) Kinetic and isotherm studies on ciprofloxacin and adsorption using magnesium oxide nanoparticles. J Appl Pharm Sci 7(11):079–083
Li Z, Hong H, Liao L, Ackley CJ, Schulz LA, MacDonald RA (2011) A mechanistic study of ciprofloxacin removal by kaolinite. Colloids Surf B 88(1):339–344. https://doi.org/10.1016/j.colsurfb.2011.07.011
Martinez JL (2009) Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut 157:2893–2902. https://doi.org/10.1016/j.envpol.2009.05.051
Mostafapoor FK, Ahmadi SH, Balarak D, Rahdar S (2017) Comparison of dissolved air flotation process for aniline and penicillin G removal from aqueous solutions. Sci J Hamadan Univ Med Sci 23(4):360–369. https://doi.org/10.21859/hums-230410
Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG (2002) Ultrasonically activated chemotherapeutic drug delivery in a rat model. Can Res 62:7280–7283
Onyechi KK, Igwegbe CA (2018) Shelf life determination of Picralima nitida, glibenclamide, ciprofloxacin and pefloxacin using UV spectrometry physicochemical technique. Der Pharma Chemica 10:67–74
Poulopoulos S, Arvanitakis F, Philippopoulos C (2006) Photochemical treatment of phenol aqueous solutions using ultraviolet radiation and hydrogen peroxide. J Hazard Mater 129(1–3):64–68. https://doi.org/10.1016/j.jhazmat.2005.06.044
Rahdar S, Rahdar A, Igwegbe CA, Moghaddam F, Ahmadi S (2019) Synthesis and physical characterization of nickel oxide nanoparticles and its application study in the removal of ciprofloxacin from contaminated water by adsorption: equilibrium and kinetic studies. Desalin. Water Treat 141:386–393. https://doi.org/10.5004/dwt.2019.23473
Ren C, Yang B, Wu M, Xu J, Fu Z, Guo T (2010) Synthesis of Ag/ZnO nanorods array with enhanced photocatalytic performance. J Hazard Mater 9(1):123–182. https://doi.org/10.1016/j.jhazmat.2010.05.141
Shemer H, Kunukcu YK, Linden KG (2006) Degradation of the pharmaceutical metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere 63(2):269–276. https://doi.org/10.1016/j.chemosphere.2005.07.029
Shiying Y, Ping W, Xin Y, Guang W, Zhang W, Liang S (2009) A novel advanced oxidation process to degrade organic pollutants in wastewater: microwave-activated persulfate oxidation. J Environ Sci 21(9):1175–1180. https://doi.org/10.1016/s1001-0742(08)62399-2
Ta N, Hong J, Liu T, Sun C (2006) Degradation of atrazine by microwave-assisted electrodeless discharge mercury lamp in aqueous solution. J Hazard Mater 138(1):187–194. https://doi.org/10.1016/j.jhazmat.2006.05.050
Taimoory SM, Trant JF, Rahdar A, Aliahmad M, Sadeghfar F, Hashemzaei M (2017) Importance of the inter-electrode distance for the electrochemical synthesis of magnetite nanoparticles: synthesis, characterization, computational modelling, and cytotoxicity. E-J Surf Sci Nanotechnol 15:31–39. https://doi.org/10.1380/ejssnt.2017.31
Zhang CL, Qiao GL, Zhao F, Wang Y (2011) Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. J Mol Liq 163:53–56. https://doi.org/10.1016/j.molliq.2011.07.005
Acknowledgements
The authors wish to express their gratitude to the laboratory staff of the Department of Environmental Health Engineering, Zabol University of Medical Sciences, for their financial support and their collaboration during this research.
Funding
The research was financially supported by the laboratory staff of the Department of Environmental Health Engineering, Zabol University of Medical Sciences (Cod.96.033).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmadi, S., Osagie, C., Rahdar, S. et al. Efficacy of persulfate-based advanced oxidation process (US/PS/Fe3O4) for ciprofloxacin removal from aqueous solutions. Appl Water Sci 10, 187 (2020). https://doi.org/10.1007/s13201-020-01271-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01271-7