Abstract
Wastewater treatment is of great concern in the textile industries because dyes, which are released into wastewater, are stable due to their structures and that some of the existing conventional techniques are not effective for aqueous removal of dyes. Among the advanced techniques for treatment of industrial effluents, adsorption is one of the most effective processes for removal of dyes from industrial wastewater. Similarly, activated carbons from different sources have been successfully applied as adsorbents for dye removal from aqueous solutions. In this context, this research evaluated the performance of activated carbon produced from peach pit (APC-1.0) for removal of a textile dye, Procion Red MX-5B (PR-5B), through batch adsorption process. The characteristic features of the peach activated carbon were obtained through Fourier transforms infrared spectroscopy, and scanning electron microscopy. The effectiveness of the activated to remove PR-5B from aqua system was compared with that of commercial activated carbon (CAC). In the kinetic study, the contact time was evaluated between 0 and 480 min using two different initial concentrations. Adsorption isotherms of PR-5B onto the two adsorbents were investigated in six different temperatures (298–326 K). The thermodynamic parameters of the process indicated spontaneous and exothermic adsorption. Equilibrium data were best described by Liu isotherm model with maximum removal capacities of 297.2 and 174.0 mg g−1 for APC-1.0 and CAC, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effluents containing dyes, when released into rivers, can cause serious environmental problems. The presence of dyes in water is undesirable due to the lasting color and can cause a decrease in water transparency even at low concentrations, thereby preventing the penetration of sunlight and hindering the process of photosynthesis and increasing the chemical oxygen demand (Bae and Freeman 2007; Rosales et al. 2016). Furthermore, some dyes can cause allergies, cancer, and mutations (Alves de Lima et al. 2007; Demarchi et al. 2013).
The treatment of effluents containing dyes is one of the biggest challenges for the textile industry due to the fact that these substances have high chemical and structural stability, and are commonly persistent to the conventional wastewater treatments. Thus, it is usually necessary to apply more than one technology since each procedure has a limitation and the dyes are resistant to degradation and are photostable (Guaratini and Zanoni 2000). Among the existing methods for the treatment of textile effluents, the adsorption has several advantages such as high color removal efficiency, low cost, ease of operation, continuous processing of large volumes of waste, and high possibility of regenerating spent adsorbent (Carneiro et al. 2010; Sharma et al. 2013; Rovani et al. 2014).
Due to the high stability of dyes, adsorption is one of the most efficient treatment process due to the easiness of design, operational simplicity and good efficiency in the removal of several type of pollutants, including dyes (Ribas et al. 2014; Rosales et al. 2016; Bazzo et al. 2016). In recent years, activated carbons have been mentioned by the Environmental Protection Agency of the USA as the best available control technology for wastewater treatment (de Franco et al. 2017). Although, there is a growing interest in search for alternative low-cost adsorbents, especially those prepared from wastes (SenthilKumar et al. 2010; Baccar et al. 2012; Dorado et al. 2014).
Brazil has a great agricultural potential and in this scenario the production of a large volume of waste can represent an environmental issue. The use of agricultural wastes to produce adsorbents is advantageous and economic alternative, which can be further used for water and wastewater treatment (Ribas et al. 2014).
In the present study, homemade peach activated carbon (APC), a low-cost adsorbent, was prepared and applied for the removal of Procion red, a textile dye, through batch adsorption process. Batch experiments were performed to investigate kinetic, equilibrium and thermodynamic aspects of the adsorption process. The adsorption potential of APC was compared with that of commercial activated carbon (CAC).
Materials and methods
Preparation of the adsorbent
Peach pit was collected, dried in an oven at 105 °C for 24 h, ground in a knife mill and properly stored for later use. For comparative purposes, all adsorption experiments were also carried out using commercial activated carbon (CAC) supplied by Merck (325–400 mesh) under the same experimental conditions of the synthesized peach carbon.
Carbon synthesis
The activated carbon was prepared using the following procedure: 70 g of organic component (peach pit powder), 70 g of inorganic components (28.6% clay, 28.6% lime, 14.3% of KOH, 14.3% Al (NO3)3 and 14.2% Na2SO4), and 50.0 mL of water were thoroughly mixed to obtain a homogeneous paste (Ribas et al. 2014; Saucier et al. 2015). The resulting wet paste was placed in a cylinder of 4.8 by 14.0 cm and dried at room temperature for 24 h. The cylinder together with the paste was then placed in a stainless-steel reactor. The reactor was heated in the tubular furnace at 293 K min−1 to 1073 K and remained at that temperature for 30 min. The resulting adsorbent was cooled to ambient temperature under Argon flow (25 mL min−1), ground, sieved to a particle size ≤ 150 µm, and stored in a sealed container until used.
To remove inorganic compounds and improve the adsorption capacity of the produced activated peach carbon, chemical activation was performed on the adsorbent. The procedure used is summarized as: 10 g of the carbon was placed in contact with 200 mL of 6 mol L−1 HCl in a 500-mL boiling flask, and the mixture was stirred on a magnetic stirrer for 2 h under reflux at 70 °C. The slurry was cooled down and filtered under vacuum using 0.45 µm membranes in a polycarbonate Sartorius system. The residue, after extensive washing with water (pH 2.0), was oven-dried at 100 °C for 5 h and stored until use. The adsorbent was named activated peach carbon (APC-1.0). The APC-1.0 contain peach pit (precursor) and inorganic components in a ratio 1:1. The ratio of the precursor to inorganics was varied during preliminary experiments, however, the composition of 1:1 (precursor/inorganic) gave the highest adsorption capacity, hence was used for further studies.
Characterization activated carbon
The activated carbon from peach was characterized using Fourier transform infrared (FTIR) spectroscopy, and scanning electron microscope (SEM). The surface morphology of APC-1.0 was obtained using SEM (Hitachi, Tabletop microscope model TM3000, Tokyo, Japan). The functional groups present in APC-1.0 were visualized FTIR (Shimadzu Spectrometer, IR Prestige 21, Kyoto, Japan). For FTIR analysis, APC-1.0 and KBr were dried in an oven at 120 °C for 8 h and kept in a desiccator. The spectrum was obtained with a resolution of 4 cm−1 with 100 cumulative scans.
Preparation of the solutions
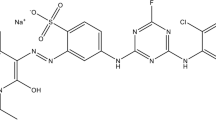
The stock solution (5000 mg L−1) of Procion Red MX-5B dye (CAS: 17804-49-8, C19H10Cl2N6O7S2, 615.33 g mol−1, Sigma-Aldrich, purity 40%, Fig. 1) was prepared by dissolving 5.0 g of the dye in deionized water. All working solutions used in the experiments were obtained by dilution of the stock solution. The pH adjustment, when needed, was performed with addition of 0.1 mol L−1 HCl or NaOH solution.
Batch studies
Batch adsorption experiments were carried out in 50 ml Falcon tubes with 20 mL of dye solution and 50 mg of adsorbent. Samples were agitated in a thermostatic shaker at 150 rpm at pre-determined times. The mixtures were then centrifuged for 10 min at 15,000 rpm. To determine the residual dye in the supernatant, the solution was analyzed by UV–VIS spectrophotometer (T90, PG Instruments, London, United Kingdom) at a wavelength of 536 nm. When necessary, aliquots were diluted with an aqueous solution (water of suitable pH) before spectroscopic measurement. The amount of adsorbed dye and the percentage removal are calculated using Eqs. 1 and 2, respectively.
where q is the amount of dye adsorbed by the adsorbent in mg g−1, C0 is the initial dye concentration in contact with the adsorbent in mg L−1, Cf is the final dye concentration after the adsorption process in mg L−1, m is the mass of adsorbent in g and V is the volume of dye solution in L.
Kinect study
For the kinetic adsorption study, the contact times were varied between 0 and 480 min using two different initial concentrations (400 and 600 mg L−1). These initial concentrations were selected because it is believed that in these conditions the saturation of the adsorbent material would be achieved.
The kinetic models used for evaluation and explanation of kinetic data were pseudo-first-order, pseudo-second-order, general-order and intra-particle diffusion models. The respective models are represented by Eqs. 3–6.
where k1 is the pseudo-first-order rate constant in min−1; k2 is the pseudo-second-order rate constant in g mg−1 min−1; kn is the nth order rate constant in min−1 (g mg−1)n−1; kid is the intra-particle rate constant in mg g−1 min−0.5, n is the order of reaction; qe is the amount of dye adsorbed by the adsorbent at equilibrium in mg g−1; and qt is the amount of dye adsorbed by the adsorbent at time, t, in mg g−1.
Equilibrium study
Experiments were performed using different dye concentrations in the range 500–1700 mg L−1 to cover a wide range of concentrations since industrial waste disposal show varying concentrations. Studies were performed at six different temperatures, which ranged between 25 and 50 °C.
The mathematical models used to describe the adsorption isotherms were Langmuir (1918), Freundlich (1906) and Liu et al. (2003) as shown in Eqs. 7–9 respectively.
Kinetic and equilibrium data were subjected to nonlinear fitting methods with successive interactions calculated by the Levenberg–Marquardt method. Interactions were also evaluated with the aids of the simplex method, based on the nonlinear fitting facilities of the Microcal Origin 9.0 software. A determination coefficient (\( R^{2} \)), an adjusted determination coefficient (\( R_{\text{adj}}^{2} \)) and an error function (\( F_{\text{error}} \)) were jointly used to evaluate the suitability of the models used (Cardoso et al. 2011; Saucier et al. 2015). A \( F_{\text{error}} \) is defined as a measure of the differences between the theoretical and experimental amounts of dye adsorbed. The \( R^{2} \), \( R_{\text{adj}}^{2} \) and \( F_{\text{error}} \) are given by Eqs. 10–12, respectively.
In these equations, \( q_{{i,{\text{model}}}} \) represents individual theoretical q value predicted by the model; \( q_{{i,{ \exp }}} \) represents individual experimental q value; \( \bar{q}_{ \exp } \) is the average of experimental q; n represents the number of experiments while p represents the number of parameters in the fitting model (Cardoso et al. 2011; dos Reis et al. 2017).
Thermodynamic study
The thermodynamic parameters related to the adsorption process, standard Gibbs energy (\( \Delta G^{\circ} \), kJ mol−1), standard enthalpy change (\( \Delta H^{\circ} \), kJ mol−1) and entropy change (\( \Delta S^{\circ} \), J mol−1 K−1), were determined using Eqs. 13–15. Equation 15 is obtained from Eqs. (13) and (14)
where \( R \) is the universal gas constant (8.314 J K−1 mol−1); \( T \) is the absolute temperature (K); \( K \) is the adsorption equilibrium constant at the isotherm that best described the equilibrium data. Different adsorption equilibrium constants can be obtained from different isotherm models (Calvete et al. 2010; Alencar et al. 2012; Cardoso et al. 2012; Dotto et al. 2012; Suksabye and Thiravetyan 2012). Thermodynamic parameters can also be calculated from the Liu equilibrium constant, Kg, as already reported (Prola et al. 2013; Adebayo et al. 2014; Ribas et al. 2014).
Microwave regeneration
The spent adsorbent was regenerated using microwave system. The regeneration system was made up of quartz reactor loaded with pre-dried carbon, through which the gas flows. The temperature was controlled by two thermocouples. The microwave oven was operated at a frequency of 2.45 GHz with variable power from 0 to 1000 W provided by a microwave generator (Sairem). Microwaves were transmitted from the generator in a waveguide made of brass (Type 340, internal dimensions of 86.943 mm). This waveguide has a refrigerated cylinder with water inside, and it was used to absorb any reflected power. Temperature, heating rates, microwave power and gas flow rate were all controlled through a computer system using software developed just for this purpose (Fidalgo et al. 2014).
The heating ramp was 5 K min−1 and N2 gas was used with a flow rate of 16 cm3 min−1. After reaching the desired temperature, the sample remained under heating for 60 min, and then the sample was cooled to room temperature. The carbon was regenerated.
Simulation of dye-house effluents
Two simulated dye-house effluents similar to industrial textile effluents at pH 2.0 were used to evaluate the adsorbent removal capacity. The synthetic dye-house effluent contained five typical dyes that are commonly used as fabric colorants and auxiliary chemicals. It is known that between 10 and 60% of synthetic dyes and almost all auxiliary chemicals remain in the dye bath (Hessel et al. 2007). The representative dyes, auxiliary chemicals and their concentrations are shown in Table 1.
Results and discussion
Characteristics of the activated peach carbon
The functional groups on the APC surface was obtained using FTIR spectroscopy. As shown in Fig. 2a, the major absorption bands present and their assignments are: 3380 cm−1 (O–H stretching vibration of alcohol), 1628 cm−1 (aromatic rings), 1103 cm−1 (C–O of alcohols and Si–O of silicates), and 789 cm−1 (C–H of aromatic out-of-plane bending). Some of these functional groups are responsible for adsorption of PR-5B dye from aqueous solution.
The SEM micrograph that shows the surface morphology of the APC-1.0 is presented in Fig. 2b. The particles of the adsorbent are aggregated. The porous and rough surface (characterized with irregular pores with different cavities) of the adsorbent is visible (Ribas et al. 2014; dos Reis et al. 2016). Chemical activation and leaching of inorganics from activated adsorbent have been found to increase the roughness, surface area and adsorption capacity of adsorbent (Ribas et al. 2014; Saucier et al. 2015; dos Reis et al. 2017).
Effect of pH
Dyes usually have one pH range in which adsorption onto a certain material is favorable. The optimum pH value depends on the characteristics of the adsorbent and the interaction between the adsorbate and the solid surface. The pH effect was evaluated between 2 and 10 using 500 mg L−1 of Procion Red solution. The results obtained for both adsorbents, APC-1.0 and CAC, are shown in Fig. 3.
As can be seen in Fig. 3, the two adsorbents exhibit similar adsorption behavior. The APC-1.0 showed maximum percentage removal of 84.6% at pH 2. With the increase of pH, the percentage removal decreased until it reached its minimum value of 64.4% at pH 10. For the commercial activated carbon (CAC), no significant variation in the percentage removal was observed between pH 2 and 5, with an average percentage removal of 75%. However, the adsorption removal decreased with the increase of pH until it reached a minimum value of 61.2% at pH 10. Adsorption capacity of cationic dyes tends to increase at relatively high pH values whereas for the anionic dyes, the adsorption process is favored at low pH (Hubbe et al. 2012). The initial pH of all dye working solutions was set at 2 for subsequent experiments.
Kinetic studies
The kinetic experimental data were analyzed using pseudo-first-order, pseudo-second-order, general-order, and intra-particle diffusion kinetic models. The nonlinear curves for both adsorbents are presented in Fig. 4. Table 2 shows the parameters obtained for the kinetic models using APC-1.0 and CAC in two different initial concentrations. As can be observed in Fig. 4 and Table 2, experimental data were best fitted by the general-order kinetic model. Compared to other kinetic models, the general-order model showed the highest values of R2adj for both APC-1.0 and CAC.
To choose a particular model that best-described adsorption process, R2adj values alone are not enough for judgement. It is therefore important to consider standard deviation (Ferror) because this parameter evaluates the difference between each data point measured experimentally and theoretical data point predicted by model (Alencar et al. 2012; Adebayo et al. 2014). Hence, Ferror values were analyzed. For the pseudo-first-order model Ferror ranged from 5.99 to 8.25 for APC-1.0 and from 6.22 and 6.52 for CAC. For the pseudo-second-order model, Ferror ranged from 2.39 to 5.16 and 4.16 to 3.55 for APC-1.0 and CAC, respectively. For the general-order kinetic model, Ferror ranged between 1.14 and 1.83 for APC-1.0 and from 3.55 to 4.16 for CAC. The observation indicates that the general-order model best explained the adsorption process of the PR-5B dye onto both activated carbons.
It is pertinent to discuss some of the parameters of general-order model, which is the best kinetic model in this study. The values of rate constant, kn, decreased for the two adsorbents when the concentration of the dye was increased. The reaction was faster when few molecules of the dye was present in the solution, as the number of molecules (concentration) increased to compete for relatively the same number of active sites, the reaction rate decreased. However, the initial rate constant, ho, increased when the concentration was increased. In another way, when the initial concentration was increased by 1.5-fold, the half-lives (the times require to attain 50% of qe) of adsorption process increased by ca. 3.9-fold and 1.4-fold for APC-1.0 and CAC, respectively. That is, the time to reach 50% of equilibrium amount increased with concentration. This observation is expected because there are more molecules of the dye at 600 mg L−1 than at 400 mg L−1, hence more time is needed for the process to be half-completed at 600 mg L−1 than at 400 mg L−1.
The results from the kinetic study and the effect of pH indicated that the PR-5B adsorption mechanism onto APC-1.0 and CAC involves ion exchange mechanisms. To confirm the existence of electrostatic attractions, thermodynamic study was conducted.
To determinate the influence of mass transfer resistance in the PR-5B adsorption on the two adsorbents, the intra-particle diffusion model was used. The graph of qt versus t1/2 is shown in Fig. 5. The adsorption of PR-5B showed three steps, which are represented by three linear portions of the curve. The first linear portion is the fastest step and it is related to the transport of dye molecules to the adsorbent surface. The second portion is a slow process and it is attributed to intra-particle diffusion. The third and final step represents the stage when the diffusion through the smaller pores decreases and the equilibrium is reached (Calvete et al. 2010; de Franco et al. 2017). This kinetic profile in three well-defined linear portions reveals that adsorption is controlled by the resistance mass transfer.
According to the data presented, the minimum contact times for equilibrium to occur between the dye and the adsorbent are 120 min for APC-1.0 and 300 min for CAC. In the equilibrium experiments, the contact time was increased to ensure that equilibrium is reached even at high concentrations of the dye (Prola et al. 2013; Ribas et al. 2014).
Adsorption isotherms
Six temperatures in the range between 25 and 50 °C were studied and the equilibrium experimental data were fitted into Langmuir, Freundlich and Liu isotherms (Table 3). Isotherms at 25 °C for the PR-5B adsorption onto APC-1.0 and CAC are shown in Fig. 6. As seen in Table 3, for all temperatures evaluated, the adsorption equilibrium of PR-5B dye was best described by Liu model because the model exhibited lowest standard deviation indices among the three models. The Ferror values of Liu model ranged from 2.75 to 6.55 for APC-1.0 and from 2.34 to 4.88 for CAC. The maximum adsorption capacities (Qmax) of PR-5B were obtained at 25 °C for both adsorbents tested—297.2 mg g−1 for APC-1.0 and 174 mg g−1 for CAC. This result revealed that the activated peach carbon had higher adsorption capacity that the commercial activated carbon under the same experimental conditions.
The best model (Liu model) for description of equilibrium data in this study, combined Langmuir and Freundlich isotherm models, however, the infinite adsorption assumption from the Freundlich model and the monolayer assumption of Langmuir model were jettisoned (Liu et al. 2003). The adsorbate molecules may prefer and occupy certain active sites on the surface of adsorbent (Liu et al. 2003; Lima et al. 2015). As shown in Table 3, the Liu equilibrium constant, Kg (L mg−1), decreases as temperature increases. This signifies that an increase in temperature does not favor the adsorption process, hence, an exothermic process. The values nL (dimensionless exponent of the Liu model) assume positive values and range from 0.8516 to 3.262 for APC-1.0 from 0.9659 to 4.055 for CAC.
Thermodynamic study
The results of thermodynamic study obtained for PR-5B adsorption onto APC-1.0 and CAC are shown in Table 4. According to Table 4, the negative values of ΔG° reveal that the adsorption onto both adsorbents occurred spontaneously. The negative values of ΔH° indicate the process was exothermic and that the adsorption process exhibited physical characteristics (physisorption) (Sun and Wang 2010; Rathod et al. 2015). Negative values of ΔS° indicate that with an increase in temperature, there is a reduction in system instability at the solid/fluid interface, i.e., the dye molecules exhibit more ordered layers on the surface of the adsorbent (Gupta et al. 2011).
Microwave regeneration
Regeneration experiments were performed on APC-1.0 in a series of adsorption–regeneration cycles and their results are shown in Table 5. The adsorption efficiency of PR-5B gradually decreased at every regeneration cycle. In each cycle, there was a loss of ca. 12% in the adsorbent mass. Thus, a decrease in regeneration efficiency occurred due to incomplete dye removal from the solid phase and reactions on the surface of the adsorbent which can reduce the number and size of the pores or even damages on the carbon structure (Guo et al. 2011; Salame and Bandosz 1999). However, in all the adsorption–regeneration cycles, the efficiencies were significant. After the first cycle, ≈ 84% of removal was obtained and after the 4th cycle the efficiency was still significant, about 40%. The results showed the microwave technique used for regeneration of the saturated adsorbent, with PR-5B, is a viable method. Microwave technique was successfully used to regenerate bamboo charcoal (Liao et al. 2013). Sun et al. (2017) also studied microwave regeneration of activated carbon and obtained high percentages of recoveries after 5 cycles with a weight loss close to 20%. The authors cited that the regeneration can be enhanced with the use of ultraviolet radiation.
Dye-house effluents
Adsorption efficiencies of activated carbon produced from peach pit (APC-1.0) and commercial activated carbon (CAC) for the removal of PR-5B were determined using synthetic effluent solutions shown in Table 1. The UV–Vis spectra of the solutions were analyzed at wavelengths between 300 and 750 nm. The absorbance bands used to quantitate the color removal efficiency of the effluents are shown in Fig. 7. The APC-1.0 adsorbent removed 94.7% of the compounds present in the effluent A and 88.4% of the effluent B while CAC removed 82.3% and 51.4% of the effluent A and B, respectively. Both adsorbents were efficient and achieved high removal percentages of the textile dye. However, for the treatment of effluent B (effluent with high concentrations of dyes and auxiliary chemicals), APC-1.0 performed significantly better than CAC. When compared with other adsorbents reported in literature, the APC-1.0 shows high effectiveness for industrial wastewater treatment (Adebayo et al. 2014; Cardoso et al. 2012; Prola et al. 2013).
Comparison of adsorption capacities of different adsorbents
It is difficult to compare the adsorption capacities of different adsorbents for removal of a dye because dye of the same chemical structure can have different commercial names depending on the manufacturers. However, Table 6 presents maximum adsorption capacities of different adsorbents from various studies at optimized conditions. Regarding the differences in the experimental conditions of the cited references, the activated peach carbon showed excellent adsorption capacity. It is observed that APC-1.0 had higher Qmax than CAC. Thus, the activated carbon reported in this work can be successfully used for the treatment of dye effluents.
Conclusions
Activated peach carbon (APC-1.0) was prepared from peach pit and its adsorption efficiency was tested for removal of a textile dye, Procion red MX-5B. Batch experiments were performed to investigate the kinetic, equilibrium and thermodynamic aspects of the study, and the results were compared with those of commercial activated carbon (CAC) using the same experimental conditions. The contact times for adsorption of PR-5B dye to reach equilibrium were 120 and 300 min for APC-1.0 and CAC, respectively, at pH 2. The adsorption process was best described by the general-order kinetic model. The intra-particle diffusion model was used to investigate the stages of adsorption, and the kinetic data showed that adsorption onto the two adsorbents exhibited three stages. In the investigation of isotherms, Liu model was the best model for description of equilibrium data at six different temperatures ranging from 25 to 50 °C. The maximum adsorption uptake was obtained at 25 °C—297.2 mg g−1 for APC-1.0 and 174 mg g−1 for CAC. Thermodynamic adsorption parameters revealed that the adsorption process was exothermic (ΔH° < 0), and spontaneous (ΔG° < 0) for both adsorbents. The negative values of ΔS° indicated that an increase in temperature reduced the system disorder. Microwave regeneration tests with APC-1.0 were successfully conducted. After four adsorption–regeneration cycles, the adsorbent exhibited satisfactory adsorption capacities. The APC-1.0 effectively removed over 88% of textile dyes from synthetic dye-house effluents while CAC removed ca. 82%.
References
Adebayo MA, Prola LDT, Lima EC, Puchana-Rosero MJ, Cataluña R, Saucier C, Umpierres CS, Vaghetti JCP, da Silva LG, Ruggiero R (2014) Adsorption of Procion Blue MX-R dye from aqueous solutions by lignin chemically modified with aluminium and manganese. J Hazard Mater 268:43–50. https://doi.org/10.1016/j.jhazmat.2014.01.005
Akar T, Arslan S, Akar ST (2013) Utilization of Thamnidium elegans fungal culture in environmental cleanup: a reactive dye biosorption study. Ecol Eng 58:363–370. https://doi.org/10.1016/j.ecoleng.2013.06.026
Alencar WS, Lima EC, Royer B, Santos BD, Silva EA, Alves CN, Alencar WS, Lima EC, Royer B, Santos BD (2012) Application of Aqai Stalks as biosorbents for the removal of the dye Procion Blue MX-R from aqueous solution. Sep Sci Technol 47:513–526. https://doi.org/10.1080/01496395.2011.616568
Alves de Lima RO, Bazo AP, Salvadori DMF, Rech CM, de Palma Oliveira D, de Aragão Umbuzeiro G (2007) Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat Res Gen Toxicol Environ 626(1–2):53–60. https://doi.org/10.1016/j.mrgentox.2006.08.002
Baccar R, Sarrà M, Bouzid J, Feki M, Blánquez P (2012) Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem Eng J 211–212:310–317. https://doi.org/10.1016/j.cej.2012.09.099
Bae JS, Freeman HS (2007) Aquatic toxicity evaluation of new direct dyes to the Daphnia magna. Dyes Pigm 73(1):81–85. https://doi.org/10.1016/j.dyepig.2005.10.015
Bazzo A, Adebayo MA, Dias SLP, Lima EC, Vaghetti JCP, de Oliveira ER, Leite AJB, Pavan FA (2016) Avocado seed powder: characterization and its application as biosorbent for crystal violet dye removal from aqueous solutions. Desalin Water Treat 57(34):15873–15888. https://doi.org/10.1080/19443994.2015.1074621
Calvete T, Lima EC, Cardoso NF, Vaghetti JCP, Dias SLP, Pavan FA (2010) Application of carbon adsorbents prepared from Brazilian-pine fruit shell for the removal of reactive orange 16 from aqueous solution: kinetic, equilibrium, and thermodynamic studies. J Environ Manag 91(8):1695–1706. https://doi.org/10.1016/j.jenvman.2010.03.013
Cardoso NF, Lima EC, Pinto IS, Amavisca CV, Royer B, Pinto RB, Alencar WS, Pereira SFP (2011) Application of cupuassu shell as biosorbent for the removal of textile dyes from aqueous solution. J Environ Manag 92(4):1237–1247. https://doi.org/10.1016/j.jenvman.2010.12.010
Cardoso NF, Lima EC, Royer B, Bach MV, Dotto GL, Pinto LAA, Calvete T (2012) Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of Reactive Red 120 dye from aqueous effluents. J Hazard Mater 241–242:146–153. https://doi.org/10.1016/j.jhazmat.2012.09.026
Carneiro PA, Umbuzeiro GA, Oliveira DP, Zanoni MVB (2010) Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J Hazard Mater 174(1–3):694–699. https://doi.org/10.1016/j.jhazmat.2009.09.106
de Franco MAE, de Carvalho CB, Bonetto MM, Soares RP, Féris LA (2017) Removal of amoxicillin from water by adsorption onto activated carbon in batch process and fixed bed column: kinetics, isotherms, experimental design and breakthrough curves modelling. J Clean Prod 161:947–956. https://doi.org/10.1016/j.jclepro.2017.05.197
Demarchi CA, Campos M, Rodrigues CA (2013) Adsorption of textile dye Reactive Red 120 by the chitosan-Fe(III)- crosslinked: batch and fixed-bed studies. J Environ Chem Eng 1(4):1350–1358. https://doi.org/10.1016/j.jece.2013.10.005
Dorado AD, Gamisans X, Valderrama C, Solé M, Lao C (2014) Cr(III) removal from aqueous solutions: a straightforward model approaching of the adsorption in a fixed-bed column. J Environ Sci Health A 49:179–186. https://doi.org/10.1080/10934529.2013.838855
dos Reis GS, Adebayo MA, Lima EC, Sampaio CH, Prola LDT (2016) Activated carbons from sewage sludge for preconcentration of copper. Anal Lett 49(4):541–555. https://doi.org/10.1080/00032719.2015.1076833
dos Reis GS, Adebayo MA, Sampaio CH, Lima EC, Thue PS, Dias SLP, de Brum AS, Pavan AF (2017) Removal of phenolic compounds using sludge-based activated carbons prepared by conventional and microwave-assisted pyrolysis. Water Air Soil Pollut 228(33):1–17. https://doi.org/10.1007/s11270-016-3202-7
Dotto GL, Lima EC, Pinto LAA (2012) Biosorption of food dyes onto Spirulina platensis nanoparticles: equilibrium isotherm and thermodynamic analysis. Bioresour Technol 103(1):123–130. https://doi.org/10.1016/j.biortech.2011.10.038
Fidalgo B, Williams HM, Dawson EA, Parkes GMB (2014) Conventional and microwave-heated oxygen pulsing techniques on metal-doped activated carbons. J Porous Mater 21(1):81–89. https://doi.org/10.1007/s10934-013-9750-y
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem A 57(25):385–471
Guaratini CCI, Zanoni MVB (2000) Corantes têxteis. Quimica Nova 23(1):71–78. https://doi.org/10.1590/S0100-40422000000100013
Guo H, Li Y, Zhao K, Ren Y, Wei C (2011) Removal of arsenite from water by synthetic siderite: behaviors and mechanisms. J Hazard Mater 186(2–3):1847–1854. https://doi.org/10.1016/j.jhazmat.2010.12.078
Gupta VK, Gupta B, Rastogi A, Agarwal S, Nayak A (2011) A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye-Acid Blue 113. J Hazard Mater 186(1):891–901. https://doi.org/10.1016/j.jhazmat.2010.11.091
Hessel C, Allegre C, Maisseu M, Charbit F, Moulin P (2007) Guidelines and legislation for dye house effluents. J Environ Manag 83(2):171–180. https://doi.org/10.1016/j.jenvman.2006.02.012
Hubbe MA, Beck KR, O’Neal WG, Sharma YC (2012) Cellulosic substrates for removal of pollutants from aqueous systems: a review. 2. Dyes. BioRes 7(2):2592–2687. https://doi.org/10.15376/biores.6.2.2161-2287
Krishni RR, Foo KY, Hameed BH (2014a) Adsorptive removal of methylene blue using the natural—banana leaves. Desalin Water Treat 52(31–33):6104–6112
Krishni RR, Foo KY, Hameed BH (2014b) Adsorption of cationic dye using a low-cost biowaste adsorbent. Desalin Water Treat 52(31–33):6088–6095
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403. https://doi.org/10.1021/ja02242a004
Liao P, Yuan S, Xie W, Zhang W, Tong M, Wang K (2013) Adsorption of nitrogen-heterocyclic compounds on bamboo charcoal: kinetics, thermodynamics, and microwave regeneration. J Colloid Interface Sci 390(1):189–195. https://doi.org/10.1016/j.jcis.2012.09.037
Lima EC, Adebayo MA, Machado FM (2015) Kinetic and Equilibrium models of adsorption. In: Bergmann CP, Machado FM (eds) Carbon nanomaterials as adsorbents for environmental and biological applications, 1st edn. Springer, New York, pp 33–69. https://doi.org/10.1007/978-3-319-18875-1_3
Liu Y, Xu H, Yang SF, Tay JH (2003) A general model for biosorption of Cd2+, Cu2+ and Zn2+ by aerobic granules. J Biotechnol 102(3):233–239. https://doi.org/10.1016/S0168-1656(03)00030-0
Noreen S, Bhatti HN, Nausheen S, Sadaf S, Ashfaq M (2013) Batch and fixed bed adsorption study for the removal of Drimarine Black CL-B dye from aqueous solution using a lignocellulosic waste: a cost affective adsorbent. Ind Crops Prod 50:568–579. https://doi.org/10.1016/j.indcrop.2013.07.065
Patel YN, Patel MP (2013) Adsorption of azo dyes from water by new poly (3-acrylamidopropyl)-trimethylammonium chloride-co-N,N-dimethylacrylamide superabsorbent hydrogel—equilibrium and kinetic studies. J Environ Chem Eng 1(4):1368–1374. https://doi.org/10.1016/j.jece.2013.09.024
Prola LDT, Machado FM, Bergmann CP, de Souza FE, Gally CR, Lima EC, Adebayo MA, Dias SLP, Calvete T (2013) Adsorption of Direct Blue 53 dye from aqueous solutions by multi-walled carbon nanotubes and activated carbon. J Environ Manag 130:166–175. https://doi.org/10.1016/j.jenvman.2013.09.003
Rathod M, Haldar S, Basha S (2015) Nanocrystalline cellulose for removal of tetracycline hydrochloride from water via biosorption: equilibrium, kinetic and thermodynamic studies. Ecol Eng 84:240–249. https://doi.org/10.1016/j.ecoleng.2015.09.031
Ribas MC, Adebayo MA, Prola LDT, Lima EC, Cataluña R, Feris LA, Puchana-rosero MJ, Machado FM, Pavan FA, Calvete T (2014) Comparison of a homemade cocoa shell activated carbon with commercial activated carbon for the removal of reactive violet 5 dye from aqueous solutions. Chem Eng J 248:315–326. https://doi.org/10.1016/j.cej.2014.03.054
Rosales E, Meijide J, Tavares T, Pazos M, Sanromán MA (2016) Grapefruit peelings as a promising biosorbent for the removal of leather dyes and hexavalent chromium. Process Saf Environ 101:61–71. https://doi.org/10.1016/j.psep.2016.03.006
Rovani S, Fernandes AN, Prola LDT, Lima EC, Santos WO, Adebayo MA (2014) Removal of Cibacron Brilliant Yellow 3G-P Dye from aqueous solutions by Brazilian peats as biosorbents. Chem Eng Commun 201(11):1431–1458. https://doi.org/10.1080/00986445.2013.816954
Salame II, Bandosz TJ (1999) Study of water adsorption on activated carbons with different degrees of surface oxidation. J Colloid Interface Sci 210:367–374. https://doi.org/10.1016/S0008-6223(03)00029-0
Saucier C, Adebayo MA, Lima EC, Prola LDT, Thue PS, Umpierres CS, Puchana-Rosero MJ, Machado FM (2015) Comparison of a homemade Bacury shell activated carbon with carbon nanotubes for food dye removal. CLEAN Soil Air Water 43(10):1389–1400. https://doi.org/10.1002/clen.201400669
SenthilKumar P, Ramalingam S, Senthamarai C, Niranjanaa M, Vijayalakshmi P, Sivanesan S (2010) Adsorption of dye from aqueous solution by cashew nut shell: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 261(1–2):52–60. https://doi.org/10.1016/j.desal.2010.05.032
Sharma M, Vyas RK, Singh K (2013) A review on reactive adsorption for potential environmental applications. Adsorption 19(1):161–188. https://doi.org/10.1007/s10450-012-9436-9
Suksabye P, Thiravetyan P (2012) Cr(VI) adsorption from electroplating plating wastewater by chemically modified coir pith. J Environ Manag 102:1–8. https://doi.org/10.1016/j.jenvman.2011.10.020
Sun CL, Wang CS (2010) Estimation on the intramolecular hydrogen-bonding energies in proteins and peptides by the analytic potential energy function. J Mol Struct: Theochem 956(1–3):38–43. https://doi.org/10.1016/j.theochem.2010.06.020
Sun Y, Zhang B, Zheng T, Wang P (2017) Regeneration of activated carbon saturated with chloramphenicol by microwave and ultraviolet irradiation. Chem Eng J 320:264–270. https://doi.org/10.1016/j.cej.2017.03.007
Acknowledgements
The authors are grateful to The National Council for Scientific and Technological Development (CNPq, Brazil), The Coordination of Improvement of Higher Education Personnel (CAPES, Brazil) and The Academy of Sciences for Developing World (TWAS, Italy) for financial support and fellowships.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest in the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ribas, M.C., de Franco, M.A.E., Adebayo, M.A. et al. Adsorption of Procion Red MX-5B dye from aqueous solution using homemade peach and commercial activated carbons. Appl Water Sci 10, 154 (2020). https://doi.org/10.1007/s13201-020-01237-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01237-9