Abstract
Coffee is globally the second largest most traded commodity after petroleum, and this has facilitated many countries to grow and produce coffee in commercial quantity. The production processes uses large volume of water which comes out as contaminated water. The presence of toxic chemicals like tannins, phenolic and alkaloids inhibits biological degradation. Microbial processes break down the organic substances released into water bodies slowly, using up the oxygen from the water (COD). As demand for oxygen needed to break down organic waste in a wastewater begins to exceed supply, a decrease in oxygen needed to combine with chemicals (COD) slowly creates anaerobic condition. The review looks at few of the current methods (physicochemical and biological) used in coffee wastewater management, their advantages and disadvantages including, high cost implication, complex operation and more time consumption among others; furthermore, the review suggests ion exchange technique as a better alternative based on its capacity to act as both an ion exchanger and absorber.
Similar content being viewed by others
Introduction
Coffee is a plant belonging to the family Rubiaceae, genus coffea. Its seeds are called coffee beans and can be processed into drinks. Although coffee lifespan may extend up to 100 years, its most productive years are from 5 to 25 and can equally grow from 5 to 25 m tall [National Coffee Association (USDA 2018)].
Coffee originated from Ethiopia and then spread across to Egypt, Yemen and Italy and all over Europe. Coffee has continuously gained a place in the world. Globally, coffee is the second largest most traded commodity after petroleum. It is mostly produced in the tropics and consumed in the temperate region. Three species of coffee dominate the international market thus; Arabica (Coffea Arabica) 70% has the highest quality in terms of taste and aroma, Robusta (Coffea Canephora) 28% has the highest caffeine content and Coffea Liberica 2% (von Enden et al. 2002).
Problem statement
Coffee processing method can be either dry or wet. The wet processing system uses large volume of water and therefore generates high volume of polluted effluent which are traditionally discharged easily into the nearby stream or river. Coffee processing by-product includes pulp with 43%, mucilage 12% and parchment 6.1%. This effluent is generally made of high concentration of organic matter and suspended solid and is highly acidic (Navitha and Kousar 2018; Sahana et al. 2018). Another major problem associated with wet coffee processing is the high amount of water required for processing approximately 15–20 L to 1 kg of coffee bean; if there is no recycling of water, then the resources themselves are at stake (Dadi et al. 2018). According to Cardenas et al. (2009), there is need to treat the coffee effluent before discharge because it was observed that the water pollution level of streams and rivers close to coffee processing plants is very high. Additionally, it has been observed that there has been minimal or no comprehensive review with regard to how coffee effluent is being managed in recent times.
Furthermore, the effluent produced by coffee processing plants is colored containing different macromolecules such as polyphenols (e.g., melanoidins) and caffeine. The coffee melanoidins consist of groups of ligand such as tannins, polysaccharides and combinations of them (Cardenas et al. 2009). These macromolecules are tough to degrade using conventional biological treatment processes and are accountable for its dark brown color (Péerez et al. 2007). Consequently, the pollution resulting from the discharge of colored effluent is responsible for its opacity, high chemical and biological oxygen demand, causing eutrophication, blocking light and affecting photosynthesis (Takashina et al. 2018; Tokumura et al. 2008; Péerez et al. 2007).
Although various physicochemical methods have been developed to remove the recalcitrant compound in CPWW, there is still need to explore other methods that can be cost effective as well. Pollutant removal techniques reported by studies include zero-valent iron treatment (Tomizawa et al. 2016) ionizing radiation, adsorption, electrochemical oxidation using steel electrodes, and, recently, chemical flocculation coupled with advanced oxidation processes (UV/H2O2, UV/O3 and UV/H2O2/O3). There has so far not been any review on the current coffee processing wastewater treatment. Although Shanthakumar (2018) attempted a mini review on coffee processing wastewater treatment (CPWW) methods, the review has failed to highlight the pros and cons of the methods mentioned, and other treatment methods were not also captured. This work hopes to fill in the gap by providing information on all current treatment techniques that has been adopted for CPWW, highlighting clearly the advantages and their disadvantages. Furthermore, this work suggests a viable and less explored method in the removal of recalcitrant compounds found in coffee processing wastewater.
Aim and objective
The aim of this paper is to present a review on the current methods of coffee effluent treatment with the sole objective of presenting the most appropriate and nascent technology that takes into consideration all parameters of concern (effectiveness, availability, affordability and ecologically friendly) for effective coffee effluent treatment.
The anatomy of a coffee berry
To have a better understanding of managing coffee processing effluent, it is very vital to understand how the organic pollution is generated.
The coffee berry outer skin is called the pericarp; beneath it there lies a thin layer of pulp (mesocarp) followed by a slimy layer called the pectin layer (parenchyma) (Tori Gay 2018). The bean itself is covered in an endocarp commonly referred to as parchment; inside the bean there is another thin layer called the silver skin (epidermis) (Fig. 1).
Coffee processing
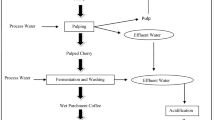
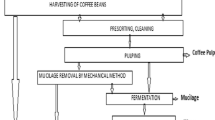
Due to the fact that the quality of coffee bean starts to degrade few hours after being picked from the farm, processing is done immediately. This is why coffee processing plants or areas should be located close to the coffee farm (Tori Gay 2018). Each technic and level of processing comes with its pollution potential (Fig. 2). Additionally, the robustness of the processing determines the quality of the end product (von Enden et al. 2002).
The dry method of coffee processing
The dry method is the simplest with minimum pollution potential. Coffee berries are left under the sun after picking until the moisture content reaches a minimum of 10%, removal of the outer skin and parchment follows suit. The dry method requires a large space for the sun drying and does not usually produce qualitative coffee; furthermore, the dry process can easily be hampered by raining season in many parts of the world (Lucy 2003).
The wet method
This method entails different stages of processing (Fig. 2) and requires high technical knowhow to ensure production of good quality coffee. The processing is preceded by sorting, where coffee berries are immersed in water. The poorly developed, degraded or diseased floats and is removed, and other detritus such as twigs or leaves that may have been collected along with berries are also removed. This is followed by depulping; it involves removing the outer layer of the coffee seed (exocarp, mesocarp), leaving a slimy coating of mucilage around the bean. This process generates high level of organic matter that makes it very difficult to degrade the mucilage layer of the coffee berries (Rattan et al. 2015). Next is fermentation of the mucilage whereby the mucilage is acted upon by microorganism in that way decomposing the layer, the coffee bean is again washed. Fermentation time is determining by the temperature of the given processing area. The simplest test for complete fermentation according to Por and Katzeff (2001) is by pushing one’s hand into the pile of coffee bean when it makes a hole and then it is completely fermented. Washing comes next after the fermentation is complete; the process involves immersing the bean in clean water, stirring and rinsing, and the process continues until it is devoid of any trace of mucilage, because having any residue of mucilage could affect the quality of the end product. After the bean has been freed from all pulpy residue, it is subjected to drying either by solar energy or by the use of mechanical dryers that are powered either electrically or fueled by gas where the moisture content is reduced to 10–12% by simply using a digital grain moisture meter for measurement (Kebede et al. 2010); the dryness of the coffee is very important because it increases the quality invariably increasing the value, and it also inhibits the growth of fungi on the seed.
Biological and physicochemical characteristics and effects of coffee processing wastewater
It has been reported by several studies that coffee wastewater is characterized by very high chemical oxygen demand (COD), biological oxygen demand (BOD), acidic content, odor and color (Tokumura et al. 2006; Chagas et al. 2015; Navitha and Kousar 2018; Gupta et al. 2009; Kebede et al. 2010; von Enden et al. 2002; Padmapriya et al. 2013, 2015; Gerardi 2003; Woldesenbet et al. 2014).
It is paramount to note that the main environmental effect of organic pollution in water is the decrease or insufficiency of oxygen and opacity (Morales et al. 2005). The presence of toxic chemicals like tannins, phenolic and alkaloids inhibits biological degradation or organic materials in water bodies where the effluent has been discharged. Microbial processes break down the organic substances released into water bodies slowly, using up the oxygen from the water. As demand for oxygen begins to exceed supply, decrease in oxygen content slowly creates anaerobic condition (Rattan et al. 2015; Tokumura et al. 2008).
This phenomenon results in very high amount of oxygen needed to break down organic waste in a wastewater (BOD) and also a high amount of oxygen needed to combine with chemicals (COD). The anaerobic conditions created in wastewater are responsible for odor and could be very fatal to aquatic inhabitants; bacteria that seep into potable water sources can as well cause direct health implication on humans (Woldesenbet et al. 2014). Color is a significant factor for aquatic life in the manufacture food from sun rays. The photosynthetic activity decreases due to dark color. This in turn will affect other parameters like temperature, DO and BOD (Siddiqui and Waseem 2012)
In general, the need for a sustainable wastewater management practices for industries is driven by many factors including legislation, disposal limits in sensitive water bodies, protection from water-related risk, economic consideration with regards to cost of water, corporation image, (as a form social corporate responsibility of company or even as part corporation obligation to the environment). Therefore, this review looks at the methods that have been in use so far and provide a not-to-common technology in the management coffee processing wastewater.
Current treatment technologies and their advantages and disadvantages
For any treatment to be ascertained as effective, the method must take into cognizance operational cost, environmental friendliness as well as the different demand for water quality in relation to water uses so as to optimize the reuse based on the water-fit-for-use principle. Wastewater must also be discharged based on predetermined discharge limits. Stability and reliability of the management method should also be considered (Hubbe et al. 2016).
Having highlighted the severity of improper management of coffee effluent above, this section highlights various methods that have been applied in managing the coffee effluent with their cons and pros.
Physicochemical treatment
Physicochemical treatment methods of industrial wastewater have been preferred recently compared over biological treatment because of its ability to break complex compound in wastewater in relatively short period of time (e.g., few hours) in a controlled environment (Takashina et al. 2018; Rodrigues et al. 2014).
Zero-valent iron (ZVI) treatment
Zero-valent iron (ZVI) particulates are non-toxic, abundant and low-cost material and easily recovered by magnetism; they have in recent times been used to treat both organic and inorganic pollutants in different wastewater (Fu et al. 2014; Tomizawa et al. 2016). Their mechanism of pollution removal is reductive degradation, oxidative degradation, adsorption and precipitation. Despite their successes, spent ZVI particulates are not easily regenerated, which makes the process not economically viable as materials need to be replaced after treatment (Tomizawa et al. 2016).
Photo-Fenton method
Recently, many OAP treatments of coffee effluent have been brought into the lime light such as photo-catalysis with H2O2 and Fe2+, with ZnO (Tokumura et al. 2008). This method uses the catalytic reaction between ferrous ions in solution and hydrogen peroxide producing hydroxyl (a specie with high oxidation potential) which is the key reagent in breaking down recalcitrant compound-related lignin (Hubbe et al. 2016). Tokumura et al. (2008) used varying doses of H2O2 and UV light to decolorize coffee effluent, 93% of coffee effluent color was removed in 250 min (Kondo et al. 2010), by using combined biological and chemical treatment with the anaerobic up-flow anaerobic sludge blanket (UASB) reactor and photo-Fenton to achieve 95% BOD removal at the optimal molar concentration of H2O2 as 2.5 × 10−1 mol L−1 and that of F2+ 6.3 × 10−2 mol L−1.
Although the photo-Fenton reaction is very effective for decolorization of coffee eluent, the additional supply of hydrogen peroxide consumed in the process and the control of optimal pH at around 3.0 are drawbacks (Tokumura et al. 2006, 2008; Yamal-Turbay et al. 2012; Villanueva-Rodríguez et al. 2014).
Ultraviolet radiation (UV) catalysis (with ozone)
Ozonation has been advocated to be a better option as compared to photo-Fenton being a stronger oxidant of many organic compounds (Satori and Kawase 2014). Studies reported that the main shortcoming of ozonation is its low mineralization, but this is usually countered by UV or hydrogen peroxide (Hubbe et al. 2016). There were also claimed that UV application alone is less effective method of treatment unless it is combined with other AOP which prove that large amount of organic compound loading, such as color and turbidity were removed effectively (Takashina et al. 2018; Ashraf et al. 2016). Péerez et al. (2007) employed photo-oxidation technique using UV/H2O2 preceded by coagulation–flocculation to remove 87% COD in coffee wastewater.
Electro-oxidation
According to Hubbe et al. (2016), electro-oxidation could be defined as a process where voltages and other factors in an electrode are optimized for in-situ generation of oxidizing and reactive species. In this method, electrolysis produces different oxidative radicals (e.g hydroxyl, chlorine,) as organic compounds directly degrade on the electrode. In this method, electrolysis produces different oxidative radicals (e.g., hydroxyl, chlorine,) as organic compound directly degrade on the electrode.
Electro-oxidation has been studied by many researchers in the treatment of coffee processing wastewater (Ibarra-Taquez et al. 2017). Cardenas et al. (2009) used a dimensionally stable anode that is composed of titanium with a film of ruthenium, cobalt oxide and iridium to reduce the level of pollutant after pretreatment with coagulation/chemical flocculation. Bejankiwar et al. (2003) studied the removal of COD and color after biological pretreatment showing that steal anode was effective in removing COD in coffee wastewater.
Membrane filtration It is a mechanical separation technique for removing micron-sized contaminants (Park et al. 2015). The technique has been reportedly successful in the treatment of pulp and paper industrial wastewater (Hubbe et al. 2016; Puro et al. 2010; Pizzichini et al. 2005) and as co- treatment in many respect (Santhosh et al. 2016; Zheng et al. 2013). There are basically four classification of membrane filtration: microfiltration with typical pore size of 0.1–2 μm, ultrafiltration 2–100 nm, nano-filtration 1–5 nm and reverse osmosis < 1 nm (Barakat 2011; Hermosilla 2016; Sójka-Ledakowicz et al. 1998).
Despite its wide usage, the main drawback of membrane filtration is that only it separates and does not change any components of the wastewater. Therefore, in the case of highly polluted wastewater such as coffee processing wastewater additional treatment method is needed as reported by Tacias-Pascacio and Torrestiana-Sanchez (2019) whereby a combined system of anaerobic baffled bioreactors and membrane filtration was used to remove COD 81%, TS 72%, TSS 100%, TDS 61%; yet, its effectiveness in the removal of recalcitrant elements such as phenol that makes up the coffee color has not been reported.
Another very common issue encountered by this method is the clogging and fouling of membrane. Recent studies were tilted to finding solution to this problem factors like rate of pressure, pretreatment before subjection to membrane filtration (Qu et al. 2012) and more recently electric treatment of the membrane; others include optimization of temperature and pH. A water treatment company operating in Malaysia developed an operator-friendly pressurized membrane system membrane bioreactor plant which will be used to treat and reuse wastewater from instant coffee processing company. Although the method will effectively remove COD and free oil and grease (Asia 2012), there is no mention of the removal of acidity and color which is the major issue with coffee processing wastewater
Biological methods
Various forms of filtration have been studied in connection with bioreactors to speed up biological decomposition (Hubbe et al. 2016) bearing in mind the health and environmental effect of direct discharge of coffee effluent without treatment (Selvamurugan et al. 2010; Dinsdale et al. 1997; Rossmann et al. 2013; Fia et al. 2012). Biological treatment is widely practiced; one of the major objectives of biological treatment is the removal of BOD, and such treatment has been found to be ineffective in the removal of color or other acidic components of the coffee wastewater and processes require a long period (a few months) to completely degrade organic materials. Biological treatment includes but not restricted to aerobic/anaerobic treatment, spray irrigation, activated sludge, immobilization, enzyme treatment, the use of bioreactors (Gupta et al. 2009; von Enden et al. 2002; Navitha and Kousar 2018).
Expanded granular sludge bed (EGSB) bioreactor Several anaerobic processes have been developed and studied to treat CPWW, such as studies on the flow pattern, toxicity inhibition, start-up, optimization, kinetics and operation conditions. The hydraulic retention time (HRT) is one of the most important independent variables for the control of this kind of anaerobic bioreactor, because it directly influences the anaerobic digestion processes and the hydrogen transfer and can produce the cell washing phenomenon (Cruz-Salomón et al. 2017).
It has been established that this type of bioreactor is an excellent system for treating high organic load effluent, but its major drawback is it does not remove the nutrients (N and P). Another problems of the anaerobic biological degradation are associated with the high content of fermentable organic matter in the effluent that causes a fast acidification of the wastewater resulting in a high production of VFA (Somasiri et al. 2008 and Buzzini et al. 2007).
Therefore, it is necessary to monitor the content of VFA for the good performance of the anaerobic bioreactor. Therefore, it is necessary to monitor the content of VFA for the good performance of the anaerobic bioreactor as well as Hydraulic retention time (HRT) because these factors affect EGSB bioreactor performance (Cruz-Salomón et al. 2017).
Chemical coagulation and flocculation is also reported to be efficient, easy to handle and cheap method of coffee wastewater treatment. It does not require pretreatment (Novita et al. 2012). Studies reviewed showed that although color removal was effective, COD reduction was not effectively achieved due to the fact that the process cannot break the complex binding of organic matter in coffee wastewater (Novita et al. 2012).
The settling down of solid particles in effluent as a result of gravity can be enhanced by electrochemical coagulation. Electrochemical coagulation method has been credited to be efficient in the removal of color, COD and SS in coffee wastewater (Novita et al. 2012; Panchangam and Janakiraman 2015; Sahana et al. 2018). Removal of suspended solid and oxygen demand occurs when high charged cation agent such as aluminum sulfate coagulant is used to neutralize to neutralize negatively charged wastewater solid, followed by high-mass polyelectrolyte such as acrylamide (flocculants) (Hubbe et al. 2016).
Electrochemical method consists of two conductive metal plates (anode and cathode) connected to an external power source where oxidation corrodes the anode material and the cathode is subjected to passivation (Sahana et al. 2018). In relation to other traditional methods of coffee processing wastewater treatment, electroplating has more advantages which include high ability to remove contaminants that are generally difficult to remove, cost effectiveness, environmental compatibility, energy efficiency, safety (Sahana et al. 2018).
Downside is noted in the gradual reduction of the effectiveness of the treatment by the passivation of cathode as current continues to flow through it. It also requires higher rate of energy supply in relation to most treatment technologies. Furthermore, the presence of tannins apparently causes a short circuit in the coagulation and the flocculation process (Novita et al. 2012).
Adsorption In the quest for cheaper and yet efficient technology for the removal of organic matter, several agricultural by-products such as corn stalk, rice husk, chitosan, chitin, peat, wood have been used as adsorbents (Devi et al. 2008). Due to their hydrophobicity, suitably high surface area and affinity, activated carbon products have been used in industrial wastewater treatment. Activated carbon technology has shown great efficiency in the removal of contaminant with very cheap and readily available adsorbent than many conventional methods of organic wastewater treatment method (Devi et al. 2008).
Although there are very few studies that adopted the adsorption technology in the management of coffee effluent, many studies have reported the use of coffee by-products as effective adsorbent in the removal of heavy metals (Anastopoulos et al. 2017; Jeguirim et al. 2017; Nitayaphat 2017; Utomo and Hunter 2006). However, Mahesh et al. (1999) reported the study of tannin removal by adsorption from coffee wastewater, and they discovered that the process effectiveness was highly dependent on pH (e.g., 3–5 using activated coconut shell and 5–6 for commercial activated carbon) even though the method has some disadvantages associated with additional cost in relation to high energy needed to activate the carbon (Shawwa et al. 2001).
Ionization irradiation
Aguilera and Consuegra (1998) studied the implication of ionizing radiation on coffee effluent organic substances that could not degrade by biological treatment alone. The method was preceded by chemical treatment. Although the method is safe, fast and effective and it does not generate any pollution, its downside has the high cost associated with the treatment method.
Advantages and disadvantages of CPWW treatment techniques
Methods | Advantages | Disadvantages | Application |
|---|---|---|---|
Zero-valent iron (ZVI) | Effective in removing color and TOC | Materials need replacement after every treatment | Tomizawa et al. (2016) |
Photo-Fenton | Efficient in removing color in coffee wastewater | Additional supply of hydrogen peroxide is required. There is also a very low pH at which the process does not neutralize. It is also time-consuming | Tokumura et al. (2008) |
Oxidation | Relatively low cost, the highest treatment percentage obtained was 75.99% for caffeic acid | Short catalytic lifespan of the enzyme when the polymerization process is not active Enzyme activity is dependent on the temperature, which makes the process cumbersome | |
Fungal species | Fungi have high potential of removing physicochemical parameters | Takes longer time for the fungi to effect considerable result. Low tolerance for fungal species to changes in pH level | |
Spray irrigation | Low cost and does not require special skill | The process did not put into consideration the fact that ground water can be confined and unconfined. Additionally, it may take longer time for pollutant to leach into ground water. Furthermore, it could also result in anaerobiosis | Loehr et al. (1988), Gupta et al. 2009 and Kebede et al. (2010) |
Aerobic/anaerobic digestion | It is the most popular treatment method | This process is time-consuming as bacterial consortia responsible for the degradation process require time to adapt to the new environment before they start to consume organic matters to grow; in addition another disadvantage of this method is the fact that the volume and strength of the effluent may not be consistent and therefore inhibit the process and the required efficiency to treat the effluent. Finally, this method will require a further post-treatment to meet the environmental standard for disposal | Padmapriya et al. (2013, 2015), von Enden et al. (2002) and Gerardi (2003) |
Coagulation | Achieved 71.9% and 97.8% of COD color removal, respectively, with 7.5 g/L of FeCl3 at pH5 | The final result has a low pH which is another problem to disposal | Novita et al. (2012) |
Activated carbon | Avocado removed 98.2 and 99.1 of COD and BOD, while commercial activated carbon 99.02% and 99.35%, respectively. The most effective method of removing COD and color | Requires huge amount of energy | Devi et al. (2008) |
Membrane filtration | Very useful as a co treatment method | Clogging and fouling of membrane, as well as required pressure | Hermosilla (2016) |
Electrochemical coagulation | Very effective for the recovery and reuse of sludge (64–85%) color removal @ pH 6 with ferrous sulfate | Requires high level of expertise, is not cost effective to small scale coffee manufacturers not efficient in color removal. Factors affecting this process include electrode material, characteristics of wastewater | |
Using Gamma radiation | The method is safe, fast and effective and it does not generate any pollution | Irradiation is a high-cost treatment | Aguilera and Consuegra (1998) |
Ion exchange
Color and organic matter removal from coffee and other colored effluents have been relatively successful; especially the physicochemical methods mentioned earlier, for these treatments to be generally considered effective, factors such as environmental friendliness demand for energy and cost of operation should also be considered. Ion exchange is the redistribution of ions between two phases of diffusion. Ion exchangers are solid materials that can take up charged ions from a solution and discharge a corresponding amount of the other ions into the solution (Kremer 1954). The ability to exchange ions is dependent on the property of the structure of the solid material (Lucy 2003; Inglezakis and Poulopoulos 2006).
Ion exchange resins are insoluble polymer that comprise basic or acidic functional groups and have the capacity to exchange counter ions within an aqueous solutions surrounding them. Ion exchange is an adsorption phenomenon where the mechanism is electrostatic. Electrostatic forces adsorb ions to charge functional groups of the ion exchange resin, and the adsorbed ion replaces the ions on the surface of the resin on a 1:1 charge ration basis (Bashir et al. 2010). The resins are fabricated from an organic polymer substrate backbone; they are usually white or yellowish available in the form of small beats (1–2 mm). The beats are generally porous providing a high surface area to maintain electro-neutrality. All ions exchangers have fixed ionic group that are balanced by the counter ion (Eq. 1) (Noble and Terry 2004):
One prominent importance of ion exchange material is the ability to use and reuse the material (Wheaton and Lefevre 1981). Ion exchange technology is utilized in many capacities such as agriculture, food processing, medical research and chemical synthesis and mostly used in water treatment processes. Their applicability to water softening, environmental remediation, wastewater treatment, hydrometallurgy, chromatography, biomolecular separations and catalysis has been highlighted in several publications (Alexandratos 2009; Silva et al. 2018). For the basis of this study, we shall be looking at their applicability in water treatment
Ion exchange techniques have been found to successfully remove heavy metals such as clinoptolite and heulandite to remove strontium (Chernjatskaja 1988); using hexacyanoferrate and phenolic resins to remove Cesium (Harjula et al. 1994; Samanta et al. 1992), using titanate exchangers to treat nuclear waste (Dosch et al. 1993), using zeolite for thorium ions removal (Sinha et al. 1994). Successes with ion exchange technique have also been recorded in recent times with regard to the treatment of various kinds of contaminated water. For examples, Bashir et al. (2010) effectively employed ion exchange technology to successfully remove color COD and ammonia nitrate in a field leachate and also for removal of ammonia from wastewater (Jorgensen and Weatherley 2003). Its advantage is seen in its capacity to remove all ionizable and ionic species from aqueous solution. It is highly efficient, and there is availability of large variety of resins that can easily be reused and recycled. The disadvantage of ion exchange resin technique is that it requires pre-filtration process before it can be used.
Advocacy of ion exchange resins for the treatment of CPWW is due to the physicochemical characteristics of ion exchanger. Major color compounds are anionic (R‐COO‐H+) from degraded plant tissue. Their chemical structure is polyphenol structure with COOH residue with molecular weight of more than 10,000 (i.e., humic acid) which can be removed by strong base anion. Meanwhile, minor color compounds are cationic (RNH3+) which can be originated from organic compound with amine residue. The color compounds also can presence with COOH group (i.e., degraded protein) and therefore could be considered as both cationic and anionic in nature; depending on pH. This compound can be removed by strong acid cation. Mechanism of pollutant removal can be by both ion exchange and physical adsorption as illustrated below

.
The ion exchange process (polar attraction) Interaction between positively charged quaternary ammonium functional group of resin and negatively charged carboxylic or sulfonic group of pollutant.
Physical adsorption (nonpolar attraction) Involves van der Waals interactions between the nonionic and the ion exchanger polystyrene matrix.
Conclusion
In conclusion, the coffee manufacturing industry plays a huge role in the world today both as a business venture and as a consumable commodity, yet the processing of a good quality coffee comes at a price. For adequate environmental, resource management and sustainability, the use of ion exchange process in the removal or organic pollutant in wastewater is highly recommended bearing in mind the successes recorded in other field similar to coffee wastewater over the years. T now, there has not been any report on the use of ion exchangers in coffee processing wastewater management. It is therefore recommended that researchers should consider this treatment process for cheap, effective as well as environmentally friendliness.
References
Aguilera Y, Consuegra R (1998) Treatment of coffee wastewater by gamma radiation. IAEA 350(15):217–220. Retrieved from http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/29/050/29050422.pdf
Alexandratos SD (2009) Ion-exchange resins: a retrospective from industrial and engineering chemistry research. Ind Eng Chem Res. https://doi.org/10.1021/ie801242v
Anastopoulos I, Karamesouti M, Mitropoulos AC, Kyzas GZ (2017) A review for coffee adsorbents. J Mol Liq 229:555–565. https://doi.org/10.1016/j.molliq.2016.12.096
Ashraf MI, Ateeb M, Khan MH, Ahmed N, Mahmood Q (2016) Integrated treatment of pharmaceutical effluents by chemical coagulation and ozonation. Sep Purif Technol 158:383–386. https://doi.org/10.1016/j.seppur.2015.12.048
Asia S (2012) MBR cleans wastewater generated during instant coffee production. Membr Technol 2012(3):2. https://doi.org/10.1016/s0958-2118(12)70046-0
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4(4):361–377. https://doi.org/10.1016/j.arabjc.2010.07.019
Bashir MJK, Aziz HA, Yusoff MS, Aziz SQ, Mohajeri S (2010) Stabilized sanitary landfilled leachate treatment using ionic resin: treatment optimization by response surface methodology. J Hazard Mater 182:115–122
Bejankiwar RS, Lokesh KS, Gowda TP (2003) Colour and organic removal of biologically treated coffee curing weastewater by electrochemical oxidation method. J Environ Sci 15(3):323–327
Cardenas A, Zayas T, Morales U, Salgado L (2009) Electrochemical oxidation of wastewaters from the instant coffee industry using a dimensionally stable RuIrCoOx anode. ECS Trans 20(1):291–299. https://doi.org/10.1149/1.3268397
Chagas PMB, Torres JA, Silva MC, Corrêa AD (2015) Immobilized soybean hull peroxidase for the oxidation of phenolic compounds in coffee processing wastewater. Int J Biol Macromol 81:568–575. https://doi.org/10.1016/j.ijbiomac.2015.08.061
Chernjatskaja NB (1988) Strontium removal by clinoptilolite and heulandite. Radiochemistry 27:618
Cruz-Salomón A, Ríos-Valdovinos E, Pola-Albores F, Lagunas-Rivera S, Meza-Gordillo R, Ruíz-Valdiviezo VM (2017) Evaluation of hydraulic retention time on treatment of coffee processing wastewater (CPWW) in EGSB bioreactor. Sustainability (Switzerland) 10(1):83. https://doi.org/10.3390/su10010083
Dadi D, Mengistie E, Terefe G, Getahun T, Haddis A, Birke W, Beyene A, Luis P, Van der Bruggen B (2018) Assessment of the effluent quality of wet coffee processing wastewater and its influence on downstream water quality. Ecohydrol Hydrobiol 18(2):201–211. https://doi.org/10.1016/j.ecohyd.2017.10.007
Devi R, Singh V, Kumar A (2008) COD and BOD reduction from coffee processing wastewater using Avacado peel carbon. Biores Technol 99(6):1853–1860. https://doi.org/10.1016/j.biortech.2007.03.039
Dinsdale RM, Hawkes FR, Hawkes DL (1997) Comparison of mesophilic and thermophilic upflow anaerobic sludge blanket reactors treating instant coffee production wastewater. Water Res 31:163–169
Dosch RG, Brown NE, Stephens HP, Anthony RG (1993) Treatment of liquid nuclear wastes with advanced forms of titanate ion exchangers. In: Waste management’93, vol 2 (Proceedings of the international symposium, Tucson, 1993), Arizona Board of Regents, Phoenix, AZ, 1751
Fia FRL, Matos AT, Borges AC, Fia R, Cecon PR (2012) Treatment of wastewater from coffee bean processing in anaerobic fixed bed reactor with different support materials: performance and kinetic modeling. J Environ Manag 108:14–21
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205
Gerardi MH (2003) The microbiology of anaerobic digesters. Wiley-Interscience, New Jersey, pp 51–57
Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39(10):783–842. https://doi.org/10.1080/10643380801977610
Harjula R, Letho J, Tusa EH, Paavola A (1994) Industrial scale removal of cesium with hexacyanoferrate exchanger—process development. Nuclear Technol 107(3):272–278
Hermosilla D (2016) Wastewater treatment and reclamation: a review of pulp and paper industry practices and opportunities. BioResources 11(August):1–139. https://doi.org/10.15376/biores.11.3.Hubbe
Hubbe MA, Metts JR, Hermosilla D, Blanco MA, Yerushalmi L, Haghighat F, Lindholm-Lehto P, Khodaparast Z, Kamali M, Elliott A (2016) Wastewater treatment and reclamation: a review of pulp and paper industry practices and opportunities. BioResources 11(3):7953–8091. https://doi.org/10.1016/j.seppur.2011.07.002
Ibarra-Taquez HN, GilPavas E, Blatchley ER, Gómez-García MÁ, Dobrosz-Gómez I (2017) Integrated electrocoagulation–electrooxidation process for the treatment of soluble coffee effluent: optimization of COD degradation and operation time analysis. J Environ Manag 200:530–538. https://doi.org/10.1016/j.jenvman.2017.05.095
Inglezakis VJ, Poulopoulos SG (2006) Adsorption, ion exchange and catalysis design of operations and environmental applications. Elsevier Academic Press, London
Jeguirim M, Limousy L, Labaki M (2017) Environmental applications of coffee processing by-products. In: Galanakis CM (ed) Handbook of coffee processing by-products: sustainable applications. Academic Press, London. https://doi.org/10.1016/B978-0-12-811290-8.00009-8
Jorgensen TC, Weatherley LR (2003) Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res 37(8):1723–1728. https://doi.org/10.1016/S0043-1354(02)00571-7
Kebede YK, Kebede T, Assefa F, Amsalu A (2010) Environmental impact of coffee processing effluent on the ecological integrity of rivers found in gomma woreda of Jimma zone, Ethiopia. Ecohydrol Hydrobiol. https://doi.org/10.2478/v10104-011-0019-2
Kondo MM, Leite KUCG, Silva MRA, Reis ADP (2010) Fenton and photo-fenton processes coupled to uasb to treat coffee pulping wastewater. Sep Sci Technol 45(11):1506–1511. https://doi.org/10.1080/01496395.2010.487451
Kremer R (1954) Ion exchange resins. France Med 17(8):16–18. https://doi.org/10.1016/S0026-0576(00)81191-5
Loehr RC, Carey WW, Kull A, Swift CA (1988) Full-scale land treatment of coffee processing waste-water. J Water Pollut Control Fed 60(11):1948–1952
Lucy CA (2003) Evolution of ion-exchange: from Moses to the Manhattan Project to modern times. J Chromatogr A 1000(1–2):711–724. https://doi.org/10.1016/S0021-9673(03)00528-4
Mahesh S, Gowda CH, Sujan RP (1999) Color tannin removal from coffee curing industrial effluents using adsorbents-IGGAC and ACS. J Pollut Res 18(1):13–19
Morales FJ, Fernadez-Fraguas C, Jimenez-Perez S (2005) Iron binding ability of melanoidins from food and model systems. Food Chem 90:821–827
Nitayaphat W (2017) Chitosan/coffee residue composite beads for removal of reactive dye. Mater Today Proc 4:6274–6283. https://doi.org/10.1016/j.matpr.2017.06.127
Navitha K, Kousar H (2018) A comparative study on the potential of Aspergillus niger and Aspergillus flavus for the treatment of coffee processing effluent. Int J Environ Ecol Fam Urban Stud 8(4):17–22
Noble RD, Terry PA (2004) Principles of chemical separations with environmental applications. https://doi.org/10.1016/S0009-2509(96)90039-1
Novita E, Bagastyo AY, Sudarjanto G (2012) Chemical coagulation of coffee wastewater for smallholder coffee agro-industry. CUTSE Conf 7:131–134
Padmapriya R, Tharian JA, Thirunalasundari T (2013) Coffee waste management—an overview. Int J Curr Sci 9:83–91
Padmapriya R, Tharian JA, Thirunalasundari T (2015) Original research article treatment of coffee effluent by Moringa oleifera seed. Int J Curr Microbiol Appl Sci 4(1):288–295
Panchangam SC, Janakiraman K (2015) Decolorization of aqueous coffee and tea infusions by chemical coagulation. Desalin Water Treat 53(1):119–125. https://doi.org/10.1080/19443994.2013.860401
Park HD, Chang IS, Lee KJ (2015) Principles of membrane bioreactors for wastewater treatment. Chapter 4. Membrane fouling. CRC Press, Boca Raton
Pizzichini A, Russo C, Di Meo C (2005) Purification of pulp and paper wastewater, with membrane technology, for water reuse in a closed loop. Desalination 178:351–359. https://doi.org/10.1016/j.desal.2004.11.045
Por Y, Katzeff P (2001) The coffee cuppers’ manifesto el manifiesto de los catadores de café. Thanksgiving coffee company, Fort Bragg
Puro L, Kallioinen M, Mänttäri M, Natarajan G, Cameron DC, Nyström M (2010) Performance of RC and PES ultrafiltration membranes infiltration of pulp mill process waters. Desalination 264(3):249–255. https://doi.org/10.1016/j.desal.2010.06.034
Qu X, Gao WJ, Han MN, Chen A, Liao BQ (2012) Integrated thermophilic submerged aerobic membrane bioreactor and electrochemical oxidation for pulp and paper effluent treatment—towards system closure. Bioresour Technol 116:1–8. https://doi.org/10.1016/j.biortech.2012.04.045
Rattan S, Parande AK, Nagaraju VD, Ghiwari GK (2015) A comprehensive review on utilization of wastewater from coffee processing. Environ Sci Pollut Res 22(9):6461–6472. https://doi.org/10.1007/s11356-015-4079-5
Rodrigues CSD, Madeira LM, Boaventura RAR (2014) Synthetic textile dyeing wastewater treatment by integration of advanced oxidation and biological processes—performance analysis with costs reduction. J Environ Chem Eng 2(2):1027–1039. https://doi.org/10.1016/j.jece.2014.03.019
Rossmann M, Matos AT, Abreu EC, Silva FF, Borges AC (2013) Effect of influent aeration on removal of organic matter from coffee processing wastewater in constructed wetlands. J Environ Manag 128:912–919
Sahana M, Srikantha H, Mahesh S, Swamy MM (2018) Coffee processing industrial wastewater treatment using batch electrochemical coagulation with SS and Fe electrodes and its combinations, recovery and reuse of sludge. Water Sci Technol 78:279–289. https://doi.org/10.2166/wst.2018.297
Samanta SK, Ramaswamy M, Misra BM (1992) Studies on cesium uptake by phenolic resins. Sep Sci Technol 27(2):255–267
Santhosh C, Velmurugan V, Jacob G, Jeong SK, Grace AN, Bhatnagar A (2016) Role of nanomaterials in water treatment applications: a review. Chem Eng J 306:1116–1137. https://doi.org/10.1016/j.cej.2016.08.053
Satori H, Kawase Y (2014) Decolorization of dark brown colored coffee effluent using zinc oxide particles: the role of dissolved oxygen in degradation of colored compounds. J Environ Manag 139:172–179. https://doi.org/10.1016/j.jenvman.2014.02.032
Selvamurugan M, Doraisamy P, Maheswari M (2010) An integrated treatment system for coffee processing wastewater using anaerobic and aerobic process. Ecol Eng 36:1686–1690
Shanthakumar SG (2018) Coffee mini review (1). Indian J Environ Prot 38(3):213–220
Shawwa AR, Smith DW, Sego DC (2001) Color and chlorinated organics removal from pulp mills wastewater using activated petroleum coke. Water Res 35(2001):745–749
Siddiqui WA, Waseem M (2012) A comparative study of sugar mill treated and untreated effluent—a case study. Orient J Chem 28(4):1899–1904
Silva RA, Hawboldt K, Zhang Y (2018) Application of resins with functional groups in the separation of metal ions/species—a review. Miner Process Extr Metall Rev 00(00):1–19. https://doi.org/10.1080/08827508.2018.1459619
Sinha PK, Amalraj RV, Krishnasamy V (1994) Studies on the ion-exchange behaviour of thorium ions with zeolites. Radiochim Acta 65(2):125–132
Sójka-Ledakowicz J, Koprowski T, Machnowski W, Knudsen HH (1998) Membrane filtration of textile dyehouse wastewater for technological water reuse. Desalination 119(1–3):1–10. https://doi.org/10.1016/S0011-9164(98)00078-2
Somasiri W, Li XF, Ruan WQ, Jian C (2008) Evaluation of the efficacy of upflow anaerobic sludge blanket reactor in removal of colour and reduction of COD in real textile wastewater. Bioresour Technol 99(9):3692–3699. https://doi.org/10.1016/j.biortech.2007.07.024
Tacias-Pascacio VG, Torrestiana-sanchez B (2019) Wastewater treatment of wet coffee processing in an anaerobic baffled bioreactor coupled to microfiltration system. Curr Environ Eng 6:45–54. https://doi.org/10.2174/2212717806666181213161302
Takashina TA, Leifeld V, Zelinski DW, Mafra MR, Igarashi-Mafra L (2018) Application of response surface methodology for coffee effluent treatment by ozone and combined ozone/UV. Ozone Sci Eng 40(4):293–304. https://doi.org/10.1080/01919512.2017.1417112
Tokumura M, Ohta A, Znad HT, Kawase Y (2006) UV light assisted decolorization of dark brown colored coffee effluent by photo-Fenton reaction. Water Res. https://doi.org/10.1016/j.watres.2006.08.012
Tokumura M, Znad HT, Kawase Y (2008) Decolorization of dark brown colored coffee effluent by solar photo-Fenton reaction: effect of solar light dose on decolorization kinetics. Water Res 42(18):4665–4673. https://doi.org/10.1016/j.watres.2008.08.007
Tomizawa M, Kurosu S, Kobayashi M, Kawase Y (2016) Zero-valent iron treatment of dark brown colored coffee effluent: contributions of a core-shell structure to pollutant removals. J Environ Manag 183:478–487. https://doi.org/10.1016/j.jenvman.2016.08.081
Tori Gay (2018) The anatomy of a coffee bean—westrock coffee. https://www.westrockcoffee.com/the-anatomy-of-a-coffee-bean/?doing_wp_cron=1536128618.8536379337310791015625. Accessed 5 Sept 2018
Torres JA, Chagas PMB, Silva MC, Dos Santos CD, Corrêa AD (2016) Enzymatic oxidation of phenolic compounds in coffee processing wastewater. Water Sci Technol 73(1):39–50. https://doi.org/10.2166/wst.2015.332
USDA (2018) Coffee: world markets and trade. Foreign Agricultural Service, pp 1–9. http://www.fas.usda.gov/psdonline/circulars/coffee.pdf. Accessed 15 Jan 2019
Utomo HD, Hunter KA (2006) Adsorption of heavy metals by exhausted coffee grounds as a potential treatment method for waste waters. E-J Surf Sci Nanotechnol 4(May):504–506. https://doi.org/10.1380/ejssnt.2006.504
Villanueva-Rodríguez M, Bello-Mendoza R, Wareham DG, Ruiz-Ruiz EJ, Maya-Treviño MDL (2014) Discoloration and organic matter removal from coffee wastewater by electrochemical advanced oxidation processes. Water Air Soil Pollut 225(12):2204
von Enden JC, Calvert KC, Sanh K, Hoa H, Tri Q, Vietnam SR, Consulting CEOR (2002) Review of coffee waste water characteristics and approaches to treatment. PPP Project, Improvement of Coffee Quality and Sustainability of Coffee Production in Vietnam. German Technical Cooperation Agency (GTZ), pp 1–10
Wheaton RM, Lefevre LJ (1981) Ion exchange. In: Kir-Othmer (ed) Encyclopedia of chemical technology, vol 13, 3rd edn. Wiley, New York
Woldesenbet AG, Woldeyes B, Chandravanshi BS (2014) Characteristics of wet coffee processing waste and its environmental impact in Ethiopia. Int J Res Eng Sci (IJRES) 2(4):1–5
Yamal-Turbay E, Graells M, Perez-Moya M (2012) Systematic assessment of the influence of hydrogen peroxide dosage on caffeine degradation by the photo-Fenton process. Ind Eng Chem Res 51:4770–4778. https://doi.org/10.1021/ie202256k
Péerez TZ, Geissler G, Hernandez F (2007) Chemical oxygen demand reduction in coffee wastewater through chemical flocculation and advanced oxidation processes. J Environ Sci 19:300–305
Zheng Y, Yu S, Shuai S, Zhou Q, Cheng Q, Liu M, Gao C (2013) Color removal and COD reduction of biologically treated textile effluent through submerged filtration using hollow fiber nanofiltration membrane. Desalination 314:89–95. https://doi.org/10.1016/j.desal.2013.01.004
Acknowledgements
Funding was provided by Universiti Sains Malaysia (Grant No. 6315062).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ijanu, E.M., Kamaruddin, M.A. & Norashiddin, F.A. Coffee processing wastewater treatment: a critical review on current treatment technologies with a proposed alternative. Appl Water Sci 10, 11 (2020). https://doi.org/10.1007/s13201-019-1091-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-1091-9