Abstract
The present study focused on the synthesis of magnetite nanoparticles in the removal of copper from aqueous solutions. Magnetite nanoparticles were developed by co-precipitation method for the adsorption of copper from aqueous solution. The characteristics of the synthesized nanoparticles were assessed using Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), field emission scanning electron microscope (SEM) and energy-dispersive X-ray (EDS) analyses. Batch adsorption experiments were performed by varying the solution pH, contact time, stirring speed, adsorbent dosage and concentration of the effluent. The magnetic nanoparticle showed an excellent performance in removing copper under the following optimized conditions: pH 2, contact time = 75 min, initial concentration of copper = 100 ppm, adsorbent dosage = 0.6 g and stirring speed = 150 RPM. Thus, the developed magnetic nanoparticle has the potential to be used for the removal of copper from aqueous solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetic nanoparticles play an important role in chemistry, physics and materials science. The utilization of magnetite nanoparticles has received much attention due to their unique properties, such as extremely small size, large surface-area-to-volume ratio, surface modifiability, excellent magnetic properties and high biocompatibility. The application of these nanoparticles will solve environmental problems to a great extent.
The growing demand for drinking water supply and effluent treatment has motivated researchers to perform extensive research in developing better water treatment technologies. Clean drinking water has become a basic need to human and also to protect public health (Junyong et al. 2018). Environmental pollution affects human and living organisms (Zhitong et al. 2012). Major metal contaminants present in water are mercury, lead, chromium, nickel, cobalt, copper, silver, arsenic, etc. These heavy metals create health and environmental issues. Various techniques employed in the removal of heavy metals from aqueous solutions are flotation, electrochemical treatments, membrane separation, adsorption, ion exchange, precipitation and biosorption processes (Shiyan et al. 2009; Yiliang et al. 2010; Ivanov et al. 2004). Among these methods, adsorption processes are most widely used technique for the removal of heavy metal ions and bacterial pathogens from water.
Development of the state-of-the-art, economical and environmentally friendly nanomaterials has gained considerable attention in recent times. Nanoparticles play a vital role in the removal of metals from wastewater (Jurgen and Joydeep 2005; Auffan et al. 2007). Magnetite nanoparticles have been extensively used in magnetic resonance imaging, ferrofluids for audio speakers, targeted drug delivery and magnetic recording (Laurent et al. 2008). In particular, magnetite nanoparticles are employed as adsorbents for the separation and removal of Zn(II) ions and other contaminants by the application of external magnetic field (Maity and Agrawal 2007; Shirsath and Shirivastava 2015). Magnetite nanoparticles are preferred in metal removal applications due to their nanosize, magnetic separation capability, simple synthesis and coating technique and surface modification (Yargeau 2012; Ratnayaka et al. 2009; Funes et al. 2018; Dias et al. 2011; Lasheen et al. 2017; Achla and Singh 2017; Ibrahim et al. 2018). Magnetic nanoparticles also find applications in drug-targeting to improve the therapeutic potential of many water-insoluble and unstable drugs (Pedro et al. 2003). Chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid were employed in the removal of Cu2+ from aqueous solution (Zhou et al. 2009). Oman is currently undergoing rapid industrialization, and this leads to a rise in the industrial effluent release. These effluents require immediate treatment so as to reduce the threat it poses to the environment.
The present study focused on the synthesis, characterization and application of magnetite nanoparticles in the removal of copper from aqueous solutions. The batch experimental study was carried out in the Chemical Engineering Laboratory of National University of Science and Technology, College of Engineering, Sultanate of Oman.
Materials and methods
Materials
The materials used for the synthesis of magnetic nanoparticles are ferrous and ferric ammonium sulphate. Magnetic nanoparticles were prepared by co-precipitation method. Stock solutions were prepared by dissolving stoichiometric amount of CuSO4 in Millipore water. All reagents were procured from Sigma-Aldrich, India.
The characterization techniques employed are field emission scanning electron microscope (SEM JEOL JSM-7600F), X-ray diffractometer (Rigaku and Mini Flex 600) and Fourier transform infrared spectroscopy (FTIR—Frontier is PerkinElmer’s). Metal ion concentration was measured by atomic absorption spectrophotometer (AAS PerkinElmer’s). All characterizations were carried out at room temperature.
The batch experiments were performed by mixing a known amount of magnetic nanoparticles into a series of conical flasks containing 100 ml of solution having a specified metal concentration. The mixture was agitated on magnetic stirrer at 25 °C for 2 h. The resulting mixture was centrifuged at 4000 RPM for 15 min, and the concentration of the supernatant was measured using atomic absorption spectrophotometer (AAS). Each experiment was repeated three times, and the average of three values is reported as the final value.
Synthesis and characterization of magnetite nanoparticles
Magnetite nanoparticles were prepared by co-precipitation method. The synthesized nanoparticles were characterized using scanning electron microscopy, FTIR, EDS and X-ray diffraction.
Adsorption studies
The adsorption studies were performed by mixing 100 ml of the copper sulphate solution with 0.1 g of magnetite particles at room temperature. The mixture was agitated using magnetic stirrer for 2 h. The resulting mixture was centrifuged at 4000 RPM for 15 min. The amount of copper adsorbed was measured using atomic absorption spectrophotometer (AAS). The effectiveness of copper adsorption from aqueous solution was studied by varying the experimental conditions such as solution pH, contact time, stirring speed, concentration of solution and dosage of magnetic nanoparticles. The percentage removal of copper was estimated using the following equation:
where Ci is the initial concentration and C0 is the final concentration in mg/l.
Effect of variation in solution pH
The effect of variation in solution pH on removal efficiency of copper was studied by changing the solution pH from 2.0 to 12.0. For this, a synthetic solution of copper sulphate was prepared and pH of the solution was varied from acidic to alkaline range. The amount of copper ions adsorbed was measured using AAS.
Effect of variation in contact time
Stirring time plays an important role in the removal of pollutants from wastewater. The effect of variation in stirring time on percentage removal of copper was studied by varying the stirring time from 30 to 75 min at optimized pH.
Effect of variation in stirring speed
The effect of variation in stirring speed on percentage removal of copper was studied by changing the stirring speed from 25 RPM to 150 RPM at optimum pH and contact time.
Effect of variation in dosage
The effect of variation in dosage of magnetic nanoparticles on percentage removal efficiency of copper was studied by varying the amount of nanoparticles from 0.1 g to 0.6 g. The other experimental conditions remain constant.
Effect of variation in the initial concentration
The effect of variation in the initial concentration of copper ion was studied by varying the solution concentration from 10 to 100 ppm, while keeping all other parameters constant. The amount of copper removal was analysed using atomic absorption spectroscopy.
Results and discussion
Synthesis and characterization of magnetic nanoparticles particles
The magnetite nanoparticles were successfully synthesized by co-precipitation method. The synthesized nanoparticles were characterized using SEM, FTIR and EDS. Figure 1 illustrates the scanning electron microscopy image of the nanoparticles at a magnification of 50,000 X. The particles were found to be very fine powder and monodispersed in nature. The particles seem to be scattered without aggregation. Figure 2 shows the EDS spectra of pure Fe3O4 nanoparticles, which confirm the presence of high-purity magnetite nanoparticles. Elemental analysis and surface coverage of magnetite particles are measured by energy-dispersive X-ray spectroscopy (SEM/EDS, EDAX).
The EDX spectral analysis shown in Fig. 2 indicates the composition of the synthesized magnetic nanoparticles. The EDS spectra presented the strong peaks of Fe and O. The composition analysis shows 62.7% Fe, 36.1% O2 and 1.2% silicon. This result demonstrates the high purity of the magnetite nanoparticles. Table 1 indicates the composition of the synthesized magnetic nanoparticles.
The functional groups present in the synthesized magnetite nanoparticles are identified by FTIR spectroscopy as shown in Fig. 3. The spectroscopic analysis indicated the absorption peaks at 530 cm−1 corresponding to the Fe–O vibration related to the magnetite phase. The peak observed at 1639 cm−1 was due to the overlapping of the absorption bands and the double bonds present in the sample.
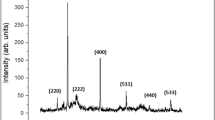
The crystal structure of the synthesized magnetite nanoparticles was assessed through X-ray diffraction (XRD) in the range of 2θ from 20° to 80°, using a diffractometer (Cu-Kα radiation, λ = 0.1541. Figure 4 shows the variation in intensity with respect to different values of theta. The patterns with the Cu were recorded in the region of 35θ range 0–1500. The maximum intensity of magnetic nanoparticles was observed at 35 degree. The positions and relative intensities of the reflection peak of magnetic nanoparticles are in agreement with the XRD diffraction peaks of standard samples.
Adsorption studies
Effect of variation in solution pH
The pH of the solution is a complex parameter since it is related to the ionization state of the nanoparticles surface and that of the copper ions present in the solution. The maximum removal efficiency of copper was observed at pH 2.0. The influence of variation in solution pH with percentage reduction is shown in Fig. 5. As shown in Fig. 5, the adsorption of copper ion is highly pH dependent. Maximum removal efficiency of 88.8% was noticed when the solution pH was 2.0 above which there is a decrease in efficiency.
Effect of variation in contact time
The stirring time at which adsorption equilibrium occurs is very important for the process optimization. The adsorption was faster at the beginning and reached 73.4% removal efficiency in the first 75 min, and thereafter, it proceeds at a lower rate, and finally, no further significant adsorption is noted beyond 1.5 h. The optimum stirring time was noticed at 75 min. In the optimized conditions, the maximum removal efficiency of copper was 73.4% (Fig. 6).
Effect of variation in stirring speed
The optimum percentage reduction is obtained when the stirring speed is 150 RPM, and almost complete removal of copper takes place when the pH is 2.0 and the stirring is continued for 75 min (Fig. 7).
Effect of variation in dosage
Dosage of magnetic nanoparticle has a great influence on the adsorption process, which determines the potential of removal efficiency through the number of binding sites available to remove copper ions at a specified solution concentration. The effect of variation in dosage of magnetic nanoparticles on copper ion removal is indicated in Fig. 8. At equilibrium, the effectiveness of copper ion removal decreases with an increase in nanoparticles dosage. This decrease can be due to the concentration gradient. The best results were obtained at 0.6 g of dosage. Effect of dosage of nanoparticles was one of the key parameters contributing to the removal efficiency. There was no considerable reduction in efficiency when the dosage was above 0.6 g. The corresponding result shows that an increase in adsorbent dosage could increase adsorption capacity. With increasing adsorbent dosage, more surface area is available for adsorption due to the increase in active sites on the surface of magnetic nanoparticles, thus making easier penetration of adsorbate to the adsorption sites.
Effect of variation in the initial concentration
The effect of variation in the initial concentration of copper ion on removal efficiency is shown in Fig. 9. The % removal of copper increased with the increase in concentration of solution. The maximum removal efficiency of copper was recorded as 41.5%.
The adsorption of copper ions was tested at lower concentrations because of their hazardous and toxic effect. Figure 4 indicates that the metal sorption capacity increased with an increase in the initial copper ion concentration. This is due to an increase in the initial ion concentration providing a larger driving force to overcome all mass transfer resistances between the solid and the aqueous phase, thus resulting in higher copper ion adsorption at the initial stages. As the concentration of copper ions in solution increases above 100 ppm, there is no further adsorption of copper due to blocking of surface area of adsorbent. This is due to the fact that the active sites on the adsorbent become saturated at higher concentrations of metal ion. Similar results have been reported (Sari and Tuzen 2010; Riaz et al. 2009; Nasuha et al. 2010; Sui et al. 2011).
Conclusions
Nanoparticles have gained remarkable attention in pharmaceuticals, catalysis and effluent treatment. Magnetite nanoparticles were successfully synthesized by co-precipitation method. The characterizations of the nanoparticles were carried out using SEM, FTIR and XRD analysis. The characterization of the synthesized nanoparticles shows good results in terms of surface morphology and scattered nature. The resulting nanoparticles were successfully employed in the removal of copper ions from aqueous solutions by performing batch adsorption studies. The optimum processing conditions obtained are pH 2.0, stirring speed of 150 RPM for a contact time of 75 min and a dosage of 0.6 g. It was found that the effect of pH has a major contribution in the removal of copper. The above results suggest the suitability of magnetic nanoparticles in the wastewater treatment applications. Currently the research team is investigating the possibility of employing surface-modified magnetite nanoparticles in the removal of pollutants from refinery, pharmaceutical and vegetable oil industry effluents.
References
Achla K, Singh SK (2017) Removal of heavy metals by nanoadsorbents: a review. J Environ Biotechnol Res 6(1):96–104
Auffan M, Shipley HJ, Yean S, Kan AT, Tomson M, Rose MJ, Bottero JY (2007) Nanomaterials as adsorbents, environmental nanotechnology: applications and impacts of nanomaterials, 3rd edn. McGraw-Hill, New York, pp 371–392
Dias AMGC, Hussain A, Marcos AS, Roque ACA (2011) A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnol Adv 29:142–155
Funes A, Martínez FJ, Alvarez-Manzaneda I, Conde-Porcuna JM, Vicente J, Guerrero F, Vicente I (2018) Determining major factors controlling phosphorus removal by promising adsorbents used for lake restoration: a linear mixed model approach. Water Res 141:377–389
Ibrahim K, Amjad K, Firdous K, Ahsan MS, Ahsanulhaq Q, Khawar SS (2018) Synthesis, characterization and applications of magnetic iron oxide nanostructures. Arab J Sci Eng 43:43–61
Ivanov V, Tay JH, Tay ST, Jiang HL (2004) Removal of micro-particles by microbial granules used for aerobic wastewater treatment. Water Sci Technol 50:147–154
Junyong Z, Jingwei H, Yatao Z, Miaomiao T, Tao H, Jindun L, Vicki C (2018) Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J Membr Sci 550:173–197
Jurgen S, Joydeep D (2005) Nanotechnology in environmental protection and pollution. Sci Technol Adv Mater 6:219–220
Lasheen M, Imam YS, Shaimaa T, Wakeel DinaY, Sabry Shahat MF (2017) Heavy metals removal from aqueous solution using magnetite Dowex 50WX4 resin nanocomposite. J Mater Environ Sci 8(2):503–511
Laurent S, Forge D, Port M, Roch A, Robic C, Vander E, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem Rev 108:2064–2110
Maity D, Agrawal DC (2007) Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J Magn Magn Mater 308:46–55
Nasuha N, Hameed BH, Mohd Din AT (2010) Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J Hazard Mater 175:126–132
Pedro T, del Puerto Morales M, Veintemillas-Verdaguer S, González-Carreño T, Serna CJ (2003) The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys 36:R182–R197
Ratnayaka DD, Brandt MJ, Johnson KM (2009) In: Ratnayaka DD, Brandt MJ, Johnson KM (eds) Water supply. Elsevier, Amsterdam, pp 315–350
Riaz M, Nadeem R, Hanif MA, Ansari TM, Rehman KU (2009) Pb(II) biosorption from hazardous aqueous streams using Gossypium hirsutum (Cotton) waste biomass. J Hazard Mater 161:88–94
Sari A, Tuzen M (2010) Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: equilibrium, thermodynamic and kinetic studies. Chem Eng J 158:200–206
Shirsath DS, Shirivastava VS (2015) Adsorptive removal of heavy metals by magnetic nanoadsorbent: an equilibrium and thermodynamic study. Appl Nanosci 5(8):927–935
Shiyan C, Yu Z, Zhiyong Y, Wei S, Shuaike S, Xiang Z, Huaping W (2009) Carboxy methylated bacterial cellulose for copper and lead ion removal. J Hazard Mater 161:1355–1359
Sui Q, Huang J, Liu Y, Chang X, Ji G, Deng S, Xie T, Yu G (2011) Rapid removal of biphenyl a on highly ordered mesoporous carbon. J Environ Sci 23:172–182
Yargeau V (2012) Water supply. In: Zeman F (ed) Metropolitan sustainability: understanding and improving the urban environment. Elsevier, Amsterdam, pp 390–405
Yiliang C, Bingcai P, Haiyan L, Weiming Z, Lu L, Jun W (2010) Selective removal of Cu(II) ions by using cation-exchange resin-supported polyethyleneimine (PEI) nanoclusters. Environ Sci Technol 44:3508–3513
Zhitong Y, Jinhui L, Henghua X, Conghai Y (2012) Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ Sci 16:722–729
Zhou YT, Nie HL, Branford White C, He ZY, Zhu LM (2009) Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. J Colloid Interface Sci 330:29–37
Acknowledgements
The authors express their sincere thanks to the Dean, National University of Science and Technology, College of Engineering, for providing the research grant. Also the authors express their sincere thanks to Associate Dean (PG & Research), National University of Science and Technology, College of Engineering, for the encouragement and moral support.
Funding
This work was supported by Caledonian Student Research Support Programme, National University of Science and Technology, College of Engineering, Oman [Project # CRS-501].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations’.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al-Jabri, M.T.K., Devi, M.G. & Al Abri, M. Synthesis, characterization and application of magnetic nanoparticles in the removal of copper from aqueous solution. Appl Water Sci 8, 223 (2018). https://doi.org/10.1007/s13201-018-0872-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0872-x