Abstract
The assessment of groundwater quality is an obligatory pre-requisite to developing countries like India with rural-based economy. Heavy metal concentration in groundwater from Besant Nagar to Sathankuppam, South Chennai was analyzed to assess the acquisition process. The study area has rapid urbanization since few decades, which deteriorated the condition of the aquifer of the area. Totally 30 groundwater samples were collected during pre-monsoon (June 2014) and post-monsoon (January 2015) from the same aquifer to assess the heavy metal concentration in groundwater. Groundwater samples were analyzed for heavy metals such as Fe, Pb, Zn, Cu, Ni, Cr, Co and Mn using atomic absorption spectrophotometry (AAS). Correlation matrix revealed that there is no significant correlation between heavy metals and other parameters during pre-monsoon except EC with Cr but Fe and Zn have good positive correlation during post-monsoon.
Similar content being viewed by others
Introduction
Recent trends show that our world is moving towards the greatest problem due to deficiency of quality and quantity of water. As a consequence, the research on quality and quantity of groundwater is in alarming stage. In Chennai, several researchers (Elango and Manickam 1987; Jayaprakash et al. 2010; Giridharan et al. 2008) and organizations like Central Ground Water Board, and Tamil Nadu Water Supply and Drainage Board are carrying out various research works using different techniques on groundwater and its aquifer by analyzing the groundwater samples and made suggestions to develop the groundwater condition of the area. When concerned about quality, even though major elements give quality index for drinking water, in particular, heavy metal concentrations are also noticed and reported because of its toxic nature. Some trace elements (Fe, Zn, Cu and Mn) are needed for biological consumption but they lead to several health issues when they are excess or deficient in water (Khan and Abbasi 2004). Some toxic elements are always having an adverse effect on humans at any dose level (lead and cadmium). Origin and source of the heavy metals in groundwater are from both point source and non-point source are reported by many authors (Sridhar et al. 2014; Kanagaraj et al. 2014). In the study area, groundwater samples are being collected during pre- and post-monsoon seasons (June 2014 and January 2015). They were analyzed for their heavy metals, such as Fe, Pb, Cd, Zn, Cu, Ni, Cr, Co and Mn. Their concentrations during pre- and post-monsoon seasons were compared. Using BIS (2012) the quality of groundwater at some locations is being warranted.
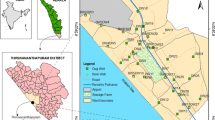
Study area
The study area is located along the coastal tract of Bay of Bengal that covers 270 sq.km and lies between 12°51′ and 12°56′30″N latitude and from 80°3′30″ to 80°14′30″E longitude (Fig. 1). The area is having a tropical climate with mean annual temperature from 24.3 to 32.9 °C. Northeast monsoon highly contributes the rainfall to the study area and the average annual rainfall is 1200 mm (CGWB 2008). Buckingham canal runs parallel to the coast and connects Muttukadu Backwater that also gives an adverse effect to the aquifer (Jayaprakash et al. 2012).
Geologically, Archean crystalline rocks are in the basement of this region, which include charnockites, and these crystalline rocks are weathered on the top which is seen in the western part of the area. Sand and some of the silt are seen along the coastal area. The thickness of the weathered rocks varies from 4 to 15 m and the thickness of the sands varies from 3 to 5 m (Brindha et al. 2014). Hydrologically, the groundwater source occurs in the shallow aquifer consisting of charnockite, sand, quartz conglomerates and recent alluvium (Fig. 2). Seawater intrusion is being reported (Elango and Manickam 1987) at some places apart from industrial effluents. The Pallikaranai marshland is an important land feature in the study area which is the home for many species such as fishes, frogs, reptiles and many birds. 60% of garbage in Chennai city is dumped in this marshland (Jayaprakash et al. 2010).
Method of sampling and analysis
Samples were collected in grid pattern, paralleling the coast 2 km internally during PRM (PRM) and POM (POM) seasons (June 2014 and January 2015). Totally, 30 samples were collected from both dug well and bore well, which are predominantly available in the area. As the groundwater occurs in shallow aquifer (< 20 m bgl), their analyses were compared together. Before sampling, high-density polyethylene sample bottles were cleaned by nitric acid. pH, electrical conductivity (µS/cm), total dissolved solid (ppm) and temperature (°C) were measured in the field itself using portable kit. Samples were immediately acidified with nitric acid to maintain the pH < 2. The concentrations of trace elements were quantitatively determined by atomic absorption spectroscopy (AAS). The results were processed in Statistical Package for the Social Sciences (SPSS) 16.0 package software for correlation analysis. The significance level (p < 0.05) based on Pearson’s correlation method done for both seasons and spatial distribution of each trace element are expressed using Arc GIS 10.1 software and the values were compared with the Bureau of Indian Standards (BIS 2012). Bureau of Indian Standards (BIS 2012) for drinking water quality are used to compare with heavy metals to estimate the groundwater quality.
Results and discussion
The minimum and maximum values of results obtained for 30 samples that were collected during pre- and post-monsoon seasons are presented in Table 1, with its BIS (2012) limit. pH value ranges from 6.63 to 8.04 in PRM and 7.3–8.8 in the POM. During PRM all the samples are within permissible limit of Bureau of Indian Standards (BIS 2012) but five samples exceed the permissible limit in POM (Fig. 3), which indicate that rainwater helps to add salts from surface to groundwater during monsoon season. The result of TDS is shown in Fig. 4. Total dissolved solids during PRM ranges from 451 to 28,840 mg/l and they range from 333 to 7850 mg/l in POM. During PRM, 28840 mg/l of TDS was observed in Besant Nagar, but during POM the value decreases to 3230 mg/l, due to continuous pumping and less precipitation after the monsoon. EC values show the same trend with TDS values, its minimum value is 644 µS/cm and the maximum is 41,200 µS/cm during PRM. During POM, 514 and 12,070 µS/cm are the minimum and maximum values, respectively.
Heavy metal distribution
Iron is an essential nutrient for human consumption. Many metabolic processes and anemia can happen due to iron deficiency in human body and it is an essential component of hemoglobin and myoglobin. Besides, it is necessary for the activity of cytochromes, peroxides, catalase, and certain other hemoprotein and flavoprotein enzymes (Khan and Abbasi 2004). In the study area, it ranges from 0.001 to 0.838 mg/l in PRM season and 0.006–1.739 mg/l in POM. Average value of Fe is 0.227 and 0.320 mg/l for both PRM and POM, respectively. During PRM, in most of the samples, it is below the acceptable limit except the sample taken from Injambakkam which shows values above the acceptable limit. Its spatial distribution is shown in Fig. 5. The trend continues during POM except for the six samples that fall within the acceptable limit. The result of increasing value of iron during POM is due to rock–water interaction (Ngah and Nwankwoala 2013). At some places, concentration of iron in groundwater is due to corrosion of household and leaching from pipes, fitting, smelting, and welding work places (WHO 2003a, b; Papachristodoulou et al. 2015).
Spatial distribution of iron in pre- and post-monsoon with BIS (2012)
Acceptable limit for lead is 0.01 mg/l (BIS 2012). For the study area, lead concentration varies from 0.112 to 0.594 mg/l, and the average is 0.247 mg/l during PRM; and during POM, it varies from 0.031 to 0.781 mg/l, and the average is 0.381 mg/l. Its spatial distribution is shown in Fig. 6. Concentration of Pb in groundwater of the study area increases during POM due to the influence of rain during monsoon that takes the Pb along with petrol fuel from roadside (WHO 2011a, b). It is also due to the release of lead-containing plumbing material including unplasticized polyvinyl chloride (uPVC) pipes that react with slight alkaline nature of water (Zhang and Lin 2014). Drinking such contaminated water is associated with hip fracture for both genders (Dahl et al. 2014), damage to the kidneys and creates hypertension for humans (WHO 2011a, b). Some of the natural organic components, such as garlic oil and vitamin E, act as detoxifying agents for lead in human body (Sajitha et al. 2010). The concentration of Cr is higher than the acceptable limit in all locations during both seasons. Its minimum and maximum concentrations were 0.229 and 0.971 mg/l during June 2014, and 0.363 and 1.484 mg/l during January 2015 (Fig. 7), respectively. Chromium is also an essential element to human, but it gives adverse effects when it exceeds its limit (WHO 2003a, b; Sutherland et al. 2000). Higher concentration of chromium in groundwater of this region is probably derived from dumping sites of municipal wastage, sewage and chrome plating industries by the influence of rainwater being precipitated during POM (Bartlett and Vesilind 1998). Due to rapid urbanization, the dumping of municipal wastes is increasing day by day in the study area. Zn is also an essential nutrient to human that is found in most of the natural foods (Umar et al. 2013) but its required and permissible limit are 5 and 15 mg/l, respectively (BIS 2012). Average value of Zn in PRM and POM is 0.054 and 0.088 mg/l, respectively. It ranges from 0.003 to 0.149 mg/l in PRM and 0.001 to 0.650 mg/l in post-monsoon (Fig. 8). Zinc concentration in all samples, in both seasons, was below the required limit of BIS (2012). Humans need a required level of Zn, otherwise it affects the metabolism and immune system and as a result human beings are susceptible to infections, delayed sexual maturation in men, and anemia and birth defects in pregnancy women (ATSDR 2005). Concentration of the zinc in groundwater is due to corrosion of household and leaching from piping, fitting, smelting, and welding workplaces (WHO 2003a, b; Papachristodoulou et al. 2015). Concentration of copper in groundwater during PRM is ranging from 0.004 to 0.112 mg/l with an average of 0.054 mg/l and during POM it is ranging from 0.001 to 0.128 mg/l with an average of 0.037 mg/l (Fig. 9). Concentration of Cu was within permissible limit in both monsoons and at the same time, most of the samples were not having the required limit for drinking purpose during both monsoons. Even though the values are within permissible limit, the higher concentrations at some places are due to agriculture activity in the area where copper leached from copper-based fertilizer and fungicide is being added to groundwater by rainwater and irrigation water (Al-Subu et al. 2003; Mirlean et al. 2009), and some concentrations are due to leaching from open dumping sites of solid wastes (Kanmani and Gandhimathi 2013), and corrosion of household materials (WHO 2004). Generally, nickel is present in Ni2+ form in natural water at a pH level of 5–9 (WHO 2005), concentration of nickel ranges from 0.093 to 0.549 mg/l with an average of 0.294 mg/l, and during the POM it ranges from 0.001 to 0.695 mg/l with an average of 0.097 mg/l (Fig. 10). PRM has higher concentration of Ni than POM. Based on BIS (2012) limit for nickel, all locations during PRM were exceeding their permissible limit and during POM, the concentration of Ni was lower than PRM but most samples were exceeding the permissible limit except at nine locations. The USEPA (1995) reported that nickel will cause body weight loss, damage of heart and liver, and dermatitis, when consumed long time above the maximum contamination level. Source of nickel in the study area is probably from sewage water and concentration from corrosion of nickel alloy materials (Purushotham et al. 2013). Concentration of cobalt values ranges from 0.085 to 0.565 mg/l during POM, and ranges from 0.273 to 0.830 mg/l during POM (Fig. 11). Spatial distribution shows that the concentration of cobalt is higher along the west portion of the study area during POM. The higher concentration of Co is due to leaching from solid waste sites in the study area (WHO 2006; Purushotham et al. 2013). Cobalt gives benefit or adverse effect to humans depending on its concentration level and types of isotope. Beneficially, it is a part of vitamin B12 and its continuous consumption above permissible limit adversely affects the heart (ASTDR 2004). There is no guideline value for cobalt in the Bureau of Indian Standards (BIS 2012). Mn concentration ranges from 0.010 to 0.518 mg/l with an average value of 0.167 mg/l in PRM, and during POM it ranges from 0.020 to 1.276 mg/l with an average value of 0.348 mg/l. Concentration of Mn is increased during POM, and during PRM most of the samples are within permissible limit, except at three locations (Fig. 12). The higher concentrations are due to rainwater being precipitated on dumping site of waste solids, which releases Mn from solid waste, from automobile emission (Loranger et al. 1994). Higher concentration of Mn will have neurological effect when continuously consumed (Zoni et al. 2007; WHO 2011a, b; Spangler and Reid 2010). As a result, the concentration of heavy metals such as Fe, Pb, Zn, Cr, Co and Mn increases in POM compared to PRM season. It indicates that the pathway of the heavy metals is that there is an accumulation of heavy metals in the soil initially when the industrial effluents and domestic dumping are spread on the ground and rainwater helps to add these metals to groundwater. Based on the Bureau of Indian Standards (BIS 2012) and average concentration of heavy metal, observed contamination level is in the order of Cr > Ni > Pb > Fe > Mn > Cu > Zn during pre-monsoon season and Cr > Pb > Ni > Mn > Fe > Cu > Zn during post-monsoon season.
Spatial distribution of lead in pre- and post-monsoon with BIS (2012)
Spatial distribution of chromium in pre- and post-monsoon with BIS (2012)
Spatial distribution of zinc in pre- and post-monsoon with BIS (2012)
Spatial distribution of copper in pre- and post-monsoon with BIS (2012)
Spatial distribution of nickel in pre- and post-monsoon with BIS (2012)
Spatial distribution of cobalt in pre- and post-monsoon with BIS (2012)
Spatial distribution of manganese in pre- and post-monsoon with BIS (2012)
Correlation matrix
The principal component analysis was based on the eigen values in the correlation matrix. The close inspection of correlation matrix was useful because it can point out associations between variables that can show the overall coherence of the data set and indicate the participation of the individual chemical parameters, a fact which commonly occurred in hydrochemistry (Helena et al. 2000). A correlation of + 1 indicates a perfect positive correlation between two variables. A correlation of − 1 indicates that one variable changes inversely with relation to the other. A correlation of zero indicates that there is no correlation between the two variables (Mudgal et al. 2009). In this study, correlation matrix was done for eight heavy metals and three field parameters. Only those with correlation values higher than 0.5 were considered (Sappa et al. 2014) for both PRM and POM seasons, respectively.
During the PRM season, there is no significant correlation between each parameter, but high negative correlation has been observed between EC and Cr with TDS (Table 2). Sources of heavy metals and other parameters are not common to any specific sources. During the POM season, highly positive correlation is observed between Fe and Zn as well as good correlation of EC with Mn. Correlation between iron and zinc indicates that they might have been derived from the same source such as corrosive activity of iron–zinc alloy of household material (WHO 2003a, 2003b; Papachristodoulou et al. 2015; Anonymous. 2016) and leachate of discharge in soil from automobile industry in POM season (Kanamar and Morita 1994) (Table 3).
Conclusion
The study identifies the seasonal variations and utility of heavy metals in groundwater. The higher pH values are noted in POM due to rainwater which adds salts from surface to groundwater during monsoon season. The samples are compared with the Bureau of Indian Standards (BIS 2012) and average concentration of heavy metal which shows contamination level in the order of Cr > Ni > Pb > Fe > Mn > Cu > Zn during POM season and Cr > Pb > Ni > Mn > Fe > Cu > Zn during POM season. The concentration of heavy metals such as Fe, Pb, Zn, Cr, Co and Mn increases in POM compared to PRM season. It indicates that the pathway of the heavy metals is that there is an accumulation of heavy metals in the soil initially when the industrial effluents and domestic dumping are spread on the ground. Correlation matrix revealed that there is no significant correlation between heavy metals and other parameters during pre-monsoon except EC with Cr, but Fe and Zn have good positive correlation during post-monsoon. The groundwater being contaminated due to rapid urbanization that causes increase of industrial effluents and dumping of domestic wastage and at the same time agriculture activities enhances the pollution to contamination level.
References
Al-Subu MM, Haddad M, Mizyed N, Mizyed I (2003) Impact of irrigation with water containing heavy metals on soil and groundwater—a simulation study. Water Air Soil Pollut 146(1–4):141–152
Anonymous (2016) Contaminants found in groundwater, USGS water science school. http://water.usgs.gov/edu/groundwater-contaminants.html. (Downloaded on 23/05/2016 at 10:30 PM) Accessed 23 May 2016
ASTDR (2004) Potential for human exposure. Agency for toxic substance and disease registry. http://www.atsdr.cdc.gov/toxprofiles/tp33-c6.pdf. Accessed 24 June 2016
ATSDR (Agency for Toxic Substances and Disease Registry) (2005) Toxicological profile for zinc. US Department of Health and Human Services, Washington
Bartlett L, Vesilind PA (1998) Expediency and human health: the regulation of environmental chromium. Sci Eng Ethics 4(2):191–201
BIS (2012) Indian standard drinking water specification (Second revision). Bureau of Indian Standards (BIS) IS 10500, New Delhi
Brindha K, Vaman KN, Srinivasan K, Babu MS, Elango L (2014) Identification of surface water–groundwater interaction by hydro geochemical indicators and assessing its suitability for drinking and irrigational purposes in Chennai, Southern India. Appl Water Sci 4(2):159–174
CGWB (2008) District groundwater brochure Chennai District, Tamil Nadu. Central Groundwater Board, India. cgwb.gov.in/District_Profile/TamilNadu/chennai.pdf
Dahl C, Søgaard AJ, Tell GS, Flaten TP, Hongve D, Omsland TK, Aamodt G (2014) Do cadmium, lead, and aluminum in drinking water increase the risk of hip fractures? A NOREPOS Study. Biol Trace Elem Res 157(1):14–23
Elango L, Manickam S (1987) Hydrochemistry of Madras aquifer, India: spatial and temporal variation in chemical quality of ground water. Geol Soc Hong Kong Bull 3:525–534
Giridharan L, Venugopal T, Jayaprakash M (2008) Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India. Environ Monit Assess 143(1–3):161–178
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res 34(3):807–816
Jayaprakash M, Urban B, Velmurugan PM, Srinivasalu S (2010) Accumulation of total trace metals due to rapid urbanization in micro tidal zone of Pallikaranai marsh, South of Chennai, India. Environ Monit Assess 170(1–4):609–629
Jayaprakash M, Nagarajan R, Velmurugan PM, Sathiyamoorthy J, Krishnamurthy RR, Urban B (2012) Assessment of trace metal contamination in a historical freshwater canal (Buckingham Canal), Chennai, India. Environ Monit Assess 184(12):7407–7424
Kanagaraj G, Sridhar SGD, Ragunathan R, Tennyson Aimol, Thabsheer KP (2014) Assessment of quality and hydro geochemistry of groundwater around sewage treatment plant at Kancheepuram Municipality, Tamil Nadu, India. Gond Geol Magz 14:149–154
Kanamar T, Morita J (1994) Properties of iron–zinc alloy-electroplated galvannealed steel sheet. T. Kanamaru, J. Morita, K. Arai (Technical Development Bureau), M. Nakayama (IN/KOTE Technology), and Y. Ogawa (Nagoya Works). Nippon Steel Tech Rep 63:23–26
Kanmani S, Gandhimathi R (2013) Investigation of physicochemical characteristics and heavy metal distribution profile in groundwater system around the open dump site. Appl Water Sci 3(2):387–399
Khan TA, Abbasi MA (2004) Effects of trace elements on human metabolism and their presence in the potable water of Ganga–Nim groundwater basin, India. Water Resour 31(5):588–591
Loranger S, Zayed J, Forget E (1994) Manganese contamination in Montreal in relation with traffic density. Water Air Soil Pollut 74:385–396
Mirlean N, Baisch P, Medeanic S (2009) Copper bioavailability and fractionation in copper-contaminated sandy soils in the wet subtropics (Southern Brazil). Bull Environ Contam Toxicol 82(3):373–377
Mudgal KD, Kumari M, Sharma DK, devMudgal K, Kumari M (2009) Hydrochemical analysis of drinking water quality of Alwar district, Rajasthan. Nat Sci 7(2):30–39
Ngah SA, Nwankwoala HO (2013) Iron (Fe2+) occurrence and distribution in groundwater sources in different geomorphologic zones of Eastern Niger Delta. Arch Appl Sci Res 5(2):266–272
Papachristodoulou C, Stamoulis K, Tsakos P, Vougidou C, Vozikis V, Papadopoulou C, Ioannides K (2015) Liver concentrations of copper, zinc, iron and molybdenum in sheep and goats from Northern Greece, determined by energy-dispersive X-ray fluorescence spectrometry. Bull Environ Contam Toxicol 94(4):460–467
Purushotham D, Rashid M, Lone MA, Rao AN, Ahmed S, Nagaiah E, Dar FA (2013) Environmental impact assessment of air and heavy metal concentration in groundwater of Maheshwaram watershed, Ranga Reddy district, Andhra Pradesh. J Geol Soc India 81(3):385–396
Sajitha GR, Jose R, Andrews A, Ajantha KG, Augustine P, Augusti KT (2010) Garlic oil and vitamin E prevent the adverse effects of lead acetate and ethanol separately as well as in combination in the drinking water of rats. Indian J Clin Biochem 25(3):280–288
Sappa G, Ergul S, Ferranti F (2014) Geochemical modeling and multivariate statistical evaluation of trace elements in arsenic contaminated groundwater systems of Viterbo Area, (Central Italy). SpringerPlus 3(1):237
Spangler JG, Reid JC (2010) Environmental manganese and cancer mortality rates by county in North Carolina: an ecological study. Biol Trace Elem Res 133(2):128–135
Sridhar SGD, Ragunathan R, Kanagaraj G, Thirusekaran C (2014) Nature of groundwater around Municipal waste treatment plant, Coimbatore, Tamilnadu, India: a study of trace element concentration. Gond Geol Magz 14:85–93
Sutherland JE, Zhitkovich A, Kluz T, Costa M (2000) Rats retain chromium in tissues following chronic ingestion of drinking water containing hexavelant chromium. Biol Trace Elem Res 74(1):41–53
Umar M, Waziri M, Hati SS (2013) Explanatory interaction profile of Cd, Pb and Zn on the relative abundance of Fe as response variable in drinking water quality assessment. Int Res J Pure Appl Chem 3(4):404–416
USEPA (1995) Technical factsheet on nickel. US Environmental Protection Agency, Washington
WHO (2003a) Chromium in Drinking-Water, Background document for preparation of WHO Guideline for drinking water quality. Geneva. World Health Organization. (WHO/SDE/WSH/03.04/4)
WHO (2003b) Zinc in Drinking-Water, Background document for preparation of WHO Guideline for drinking water quality. Geneva. World Health Organization. (WHO/SDE/WSH/03.04/17)
WHO (2004) Copper in drinking-water, background document for preparation of WHO Guideline for drinking water quality. Geneva. World Health Organization. (WHO/SDE/WSH/03.04/88)
WHO (2005) Nickel in drinking-water, background document for preparation of WHO Guideline for drinking water quality. Geneva. World Health Organization. (WHO/SDE/WSH/05.08/55)
WHO (2006) Cobalt and inorganic cobalt compounds. Concise International Chemical Assessment Document 69. World Health Organization. (ISBN 92 4 153069 3)
WHO (2011a) Lead in Drinking-Water, Background document for preparation of WHO Guideline for drinking water quality. Geneva. World Health Organization. (WHO/SDE/WSH/03.04/09/Rev/1)
WHO (2011b) Manganese in Drinking-Water, Background document for preparation of WHO Guideline for drinking water quality. Geneva. World Health Organization. (WHO/SDE/WSH/03.04/104/Rev/1)
Zhang Y, Lin YP (2014) Leaching of Lead from new unplasticized polyvinyl chloride (uPVC) pipes into drinking water. Environ Sci Pollut Res. 22(11):8405–8411. doi: 10.1007/s11356-014-3999-9
Zoni S, Albini E, Lucchini R (2007) Neuropsychological testing for the assessment of manganese neurotoxicity: a review and a proposal. Am J Ind Med 50(11):812–830
Acknowledgements
Authors are thankful to Former Head of the Department and Prof. Dr. K. K. Sharma, Department of Applied Geology, University of Madras, for providing all the support during the research period. The financial support from UGC SAP DRS II is being acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sridhar, S.G.D., Sakthivel, A.M., Sangunathan, U. et al. Heavy metal concentration in groundwater from Besant Nagar to Sathankuppam, South Chennai, Tamil Nadu, India. Appl Water Sci 7, 4651–4662 (2017). https://doi.org/10.1007/s13201-017-0628-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0628-z