Abstract
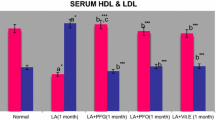
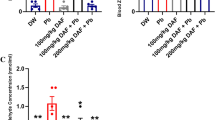
Daily feeding of drinking water containing lead acetate (160 mg/l) or 10% alcohol by volume or a combination of both to rats for a month produced certain deleterious effects through oxidative stress. Both heavy metal lead and alcohol are capable of doing such damages. The deleterious alterations observed were in the parameters of blood, serum and tissues, viz; Hb, Pb, proteins, lipids, lipid per oxidation, Vitamins C and E levels and enzyme activities of AST, ALT, and catalase. Simultaneous feeding of either of the two antioxidants garlic oil (GO) and vitamin E at equal doses of 100 mg/kg/day, to the rats counteracted the deleterious effects of the above two chemicals significantly. The maximum damage was brought about by feeding of drinking water containing both lead acetate and alcohol. The protective effects of GO and Vitamin E were not significantly different. The mechanism of actions of the Vitamin E and GO is probably due to their efficiency as detoxifying agents and antioxidants, to scavenging free radicals as well as an independent action of GO on the removal of lead salt as lead sulfide.

Similar content being viewed by others
References
Juberg DR, Klieman CF, Simons CK. Position paper of the American council on sciences and Health. Lead and human health. Ecotoxicol Environ Safety. 1997;38:162–80.
Goyer RA. Results of lead research. Prenatal exposure and neurological consequences. Environ Health Perspect. 1996;104:1050–4.
Bressler J, Kim KA, Chakraborti T, Goldstein G. Mechanism of lead neurotoxicity. Neurochem Res. 1999;24:595–600.
Flora SJS, Tandon SK. Effect of combined exposure to lead and ethanol on some biochemical indices in the rat. Biochem Pharmacol. 1987;36:537–41.
Flora SJS, Dube SN. Modulatory effects of ethanol ingestion on the toxicology of heavy metals. Ind J Pharmacol. 1994;26:240–8.
Harishekar MB. Studies on alcohol-lead interactive hepatotoxicity. Ph.D.Thesis (submitted), Rajiv Gandhi University of Health Sciences Bangalore, Karnataka. 2003. pp. 77–97.
Pet Kov V, Stoev V, Bakalov D, Petev L. The Bulgarian drug satlal as a remedy for lead intoxication in industrial conditions. Higiena Truda i Profesionaline Zabolevania. 1965;9(4):42.
Aminu B, Augusti KT, Joseph PK. Hypolipidemic effects of onion oil and garlic oil in ethanol fed rats. Ind J Biochem Biophys. 1984;21:211–3.
Augusti KT, Anuradha, Prabha SP. Nutraceutical effects of garlic oil, its non polar fraction and a ficus flavonoid as compared to Vitamin E in CC14 induced liver damage in rats. Ind J Exp Biol. 2005;43:437–44.
Augusti KT, Suneesh K, Krishnankutty BK. Prophylactic and curative effects of garlic oil as compared to α tocopherol against isoproterenol induced damages in rats In: Proceedings of the National Seminar on the Evaluation of Nutraceuticals that prevent diseases held at Thalassery Campus of Kannur University on 29th Nov–2nd Dec 2006. pp. 84–96.
Pinon-Lataillade G, Thoreux-Manlay A, Coffigny H. Effect of ingestion and inhalation of lead on the reproductive system and fertility of adult male rats and their progeny. Hum Exp Toxicol. 1993;12:165–72.
Jayaramman J editor. Modified Biuret method. In: Laboratory Manual in Biochemistry. New Delhi: Wiley Eastern Limited; 1981. p. 78.
Chiamori N, Henry R. Total cholesterol. Zlatkis (modified) method. In: Frankel S, Reitman S, Sonnenwirth AC, editors. Gradwohl’s Clinical Laboratory Methods and Diagnosis. Saint Louis: The C.V. Mosby Company; 1963. p. 257–8.
Friedewalt WT, Levy RT, Frederickson DS. Estimation of concentration of LDL in plasma with out use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Warnick RG, Albers JA. A comprehensive evaluation of the heparin-manganese precipitating procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–79.
Van Handel E, Zilversmit DB. Serum triglycerides micromethod. In: Frankel S, Reitman S, Sonnenwirth AC, editors. Gradwohl’s Clinical Laboratory Methods and Diagnosis. Saint Louis: The CV Mosby Company; 1963. p. 263–4.
Chaudhry K editor. Haemoglobin estimation by cyamethaemoglobin method. In: Biochemical Techniques. New Delhi: Jaypee Brothers Medical publishers; 1989. pp 83–85.
Tandon SK, Singh S, Prasad S. Influence of garlic on the disposition & toxicity of Lead & cadmium in the rat. Pharmaceu Biol. 2001;39:450–4.
Bergmeyer H, Bernet E, Colorimetric method for aspartatate and alanine aminotransferases. In: Varley H, Gowenlock AH, Bell M editor. Practical Clinical Biochemistry. Vol I, 5th ed. London: William Heinemann Medical Books Ltd.; 1980. p. 741742.
Maehly AC, Chance B. The assay of catalase and peroxidase. In: Glick D, editor. Methods Biochemical Analysis, vol. 1. New York: Interscience; 1954. p. 357–60.
Terada M, Watanabe Y, Kunitomo M, Hayashi E. Differential rapid analysis of AA and AAS by diphenyl hydrazine method. Anal Biochem. 1978;84:604–6.
Catiganin GL. An HPLC method for the simultaneous determination of retinol and α tocopherol in plasma or serum. Methods Enzymol. 1986;123:214–9.
Niehaus WG Jr, Samuelson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30.
Chiba M, Shinohara A, Matsushita K, Watanaba H, Inaba Y. Indices of lead exposure in blood and urine of lead-exposed workers and concentrations of major and trace elements and activities of SOD, GSH-Px and catalase in their blood. J Exp Med. 1999;178:49–62.
Sandhir R, Julka D, Gill KD. Lipoperoxidative damage on lead treatment in rat brain and its implications on membrane bound enzymes. Pharmacol Toxicol. 1994;74:66–71.
Sandhir R, Gill KD. Effects of lead on lipid peroxidation in liver of rats. Biol Trace Elem Res. 1995;48:91–7.
Lau BHS. Allergies, pollution, and today’s lifestyle. In: Garlic for Health. Wilmot, WI: Lotus Light Publications; 1988. p. 29–35.
Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318–26.
Blomstrand R, Kager L, Lantto O. Status on studies on the ethanol induced decrease of fatty acid oxidation in rats & human liver slices. Life Sci. 1973;13:113–23.
Ding Y, Gonick HC, Vaziri ND. Lead promotes hydroxyl radical generation and lipid peroxidation in cultured aortic endothetial cells. Am J Hypertens. 2001;13:552–5.
Ding Y, Gonick HC, Vaziri ND, Llang KW. Lead induced hypertension III increased hydroxyl radical production. Am J Hypertens. 2001;14:169–73.
Ellwood PC, Yarnell JWG, Oldham PD. Blood pressure and blood lead surveys in Wales. Am J Exp Demiol. 1988;127:942–5.
Barrio E, Rodriguez I, Tome S, Gude F, Quintela F. Liver disease in heavy drinkers with & without alcohol withdrawal syndrome. Alcohol Clin Exp Res. 2003;28(1):131–6.
Baily SM, Patel VB, Young TA. Chronic ethanol consumption alters the glutathione/glutathione peroxidase-1 system and protein oxidation status in rat liver. Alcohol Clin Exp Res. 2001;25:726–33.
Davies KJA. Protein damage and degeneration by oxygen radicals. J Biochem. 1987;262:9895–902.
Berlett BS, Stadtman ER. Protein oxidation in aging disease and oxidative stress. J Biol Chem. 1997;272(33):2031–6.
Humphreys DJ. Effects of exposure to excessive quantities of lead on animals. Br Vet J. 1991;147:18–30.
Juin YS, Hsien LT. Lipid peroxidation in workers exposed to lead. Arch Environ Health. 1994;49:256–9.
Farant JP, Wigfield DC. Biomonitoring of lead exposure with ALAD activity ratios. Int Arch Occup Environ Health. 1982;51:15–24.
Hermes-Lima M. How do Ca2+ and 5-aminolevulinic acid derived oxyradical promote injury to isolated mitochondria. Free Radic Biol Med. 1995;19:381–90.
Adonaylo VN, Otieza PI. Pb2+ promotes lipid peroxidation and alteration in membrane physical properties. Toxicology. 1999;132:19–32.
Pande M, Mehta A, Pant BP, Flora SJS. Combined administration of a chelating agent and an antioxidant in the prevention and treatment of acute lead intoxication in rats. Environ Toxicol Pharmacol. 2001;9:173–84.
Gurer H, Ercal N. Can antioxidant be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29:927–45.
Lawton L, Donaldson WE. Lead induced tissue fatty acid alterations and lipid peroxidation. Biol Terace Elem Res. 1991;28:83–97.
Fatma M, El-Demerdash FM. Antioxidant effect of Vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. J Trace Elem Med Biol. 2004;18:113–22.
Buettner GR. The packing order of free radicals and antioxidants. Lipid peoxidation α-tocopherol and ascorbate. Arch Biochem Biophys. 1993;300:535–43.
Patra RC, Swarup D, Dwivedi SK. Antioxidant effects of α-tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology. 2001;162:81–8.
May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha tocopherol in human erythrocytes and intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–9.
Klanns-Dieter A. Sulfur content of free radicals. In: Nygaard OF, Simic MG, editors. Radioprotectors and Anticarcinogenes. New York: Academic Press; 1983. p. 23–42.
El-Demerdash FM, Yousef MI, Abou El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan—induced diabetic rats. Food and Chem Technol. 2005;43(1):57–63.
Nuutila AM, Pimia RP, Arni M, Caldentey KMO. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003;81(4):485–93.
Onyeze GO. Effect of Vitamin E on mono sodium glutamate induced hepato toxicity, oxidative stress in rats. Ind J Biochem Biophys. 2006;43:20–4.
Babalola OO, Ojo LO, Aderemi MO. Lead levels in some biological samples of auto-mechanic in Abeokutta, Nigeria. Ind J Biochem Biophys. 2005;42:401–3.
Lau BHS. Suppression of LDL oxidation by garlic. J Nutr. 2001;131:958S–88S.
Acknowledgments
The authors acknowledge with thanks Sree Mookambika Institute of Medical Sciences and the M.G. University authorities for some financial support for the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajitha, G.R., Jose, R., Andrews, A. et al. Garlic Oil and Vitamin E Prevent the Adverse Effects of Lead Acetate and Ethanol Separately as well as in Combination in the Drinking Water of Rats. Indian J Clin Biochem 25, 280–288 (2010). https://doi.org/10.1007/s12291-010-0042-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-010-0042-x