Abstract
Hydrogeochemical investigation has been carried out in the granitic terrain of Siddipet area, Medak district, Telangana State, India with an aim to understand the distribution of fluoride in the groundwater and to understand the relationship of fluoride with other major ions, and also to identify the high fluoride-bearing groundwater zones. 104 groundwater samples were analyzed in the study area for fluoride and other major ions like calcium, magnesium, chloride, carbonate, bicarbonate, sodium, potassium, sulfate, and nitrate in addition to pH and electrical conductivity. The studies revealed that the concentration of fluoride in groundwater is ranging from 0.2 to 2.2 mg L−1 with a mean of 1.1 mg L−1. Nearly 22 % of groundwater has more than the permissible limit of fluoride (1.5 mg L−1), which is responsible for the endemic dental fluorosis in the area concerned. Geochemical classification of groundwater shows that Na–HCO3, Ca–Cl, and Ca–HCO3–Na are the dominant hydrochemical facies. Gibbs diagram shows rock–water interaction dominance and evaporation dominance, which are responsible for the change in the quality of water in the hard rock aquifer of the study area. The groundwater in villages and its environs are affected by fluoride contamination, and consequently majority of the population living in these villages suffer from dental fluorosis. Hence, they are advised to consume drinking water which has less than 1.5 mg L−1 fluoride to avoid further fluorosis risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorine is the lightest halogen and one of the most reactives of all chemical elements (Kaminsky et al. 1990). Fluorine commonly occurs as a negatively charged ion in water, either in trace amounts or as a major ion with high concentrations (Gaciri and Ad Davis 1993; Apambire et al. 1997; Fantong et al. 2009). Fluorosis is a very dangerous and deadly disease affecting millions of people across the world. More than 200 million people from all over the world (among 25 nations) suffer from endemic fluorosis, caused mainly due to excess fluoride in drinking water (Ayoob and Gupta 2006; Hong-jian et al. 2013; Moghaddam and Fijani 2008; Oruc 2008; Fordyce et al. 2007; Ghosh et al. 2013; Mesdaghinia et al. 2010).
In the two largest countries India and China of the world, fluorosis is most severe and well known. High concentration of fluoride, often above 1.5 mg L−1, constitutes a severe problem over a large part of India. About 80 % of the diseases in the world are due to the poor quality of drinking water, and the fluoride contamination in drinking water is responsible for 65 % of endemic fluorosis around the globe (Felsenfeld and Robert 1991). Furthermore, 50 % of the groundwater sources in India have been contaminated by fluoride and more than 90 % of the villages use groundwater for drinking purposes (Subarayan et al. 2012). In fact, more than 40 million people in India are affected due to the prevalence of dental fluorosis (Karthikeyan et al. 2005). In India, the excessive presence of fluorides in groundwater is noticed in nearly 177 districts covering 20 states, affecting more than 65 million people, including 6 million children (Gupta et al. 2006). The problem of excessive fluoride in groundwater in India was first reported in 1937 in the state of Andhra Pradesh (Short et al. 1937). Telangana State is one of the fluoride affected states in the country and is considered to be endemic to fluorosis.
The major health problems caused by excessive fluoride are dental fluorosis, skeletal fluorosis, and deformation of bones in children and adults (Susheela et al. 1993). Fluorosis has greatest impact on growing teeth, and children less than 7 years old are particularly vulnerable (Murray 1996). The maximum permissible limit of fluoride in drinking water is prescribed as 1.5 mg L−1 by World Health Organization and Indian Council of Medical Research (WHO 2011; ICMR 1975). Fluoride concentration in groundwater is influenced by a number of factors, such as temperature, pH, the presence or absence of complexing or precipitating ions and colloids, solubility of fluorine bearing minerals, anion exchange capacity of aquifer materials (i.e., OH− for F−), the size and type of geological formations traversed by water, and the contact time period during which water remains in contact with a particular formation (Apambire et al. 1997). There is also evidence that the adverse health effects of fluoride are enhanced by lack of Ca, vitamins, and protein in the diet (Jacks et al. 1993; Li et al. 1996). Fluorides are released into the groundwater mostly through water–rock interaction by various fluoride-bearing minerals. Fluorite (CaF2) is the sole principal mineral of fluorine occurring in nature, and is commonly found as an accessory in granitic gneiss (Ozsvath 2006; Saxena and Ahmed 2003). Fluorine is also abundant in other rock-forming minerals like apatite, micas, amphiboles, and clay minerals (Karro and Uppin 2013; Narsimha and Sudarshan 2013; Rafique et al. 2009; Naseem et al. 2010; Jha et al. 2010; Carrillo-Rivera et al. 2002).
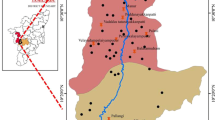
The study area is situated about 105 km north of Hyderabad on Hyderabad–Karimnagar State highway, and is bounded by E longitude 78.76942–78.90232 and N latitude 18.06768–18.24402. The area under investigation falls under semi-arid zone, with a hot, humid climate, and predominantly occupied by granite/gneiss of Archean age. The area experiences a semi-arid climate with an annual mean temperature of 30 °C. The mean annual rainfall is recorded as 745 mm, occurring mostly during the southwest monsoon period (June–September). Groundwater is the major drinking water source in the villages of Siddipet area of Medak district of Telangana State, India. Endemic fluorosis as well as its prevalence and severity are poorly known in the study area. The present study was undertaken to assess the fluoride content of groundwater and to statistically correlate the concentrations of fluoride with the other measured parameters, and also identify the wells with high F− concentration, raise awareness in people and study the water chemistry of groundwater in Siddipet area, Medak district, Telangana, India.
Materials and methods
104 groundwater samples were collected from 39 villages of Siddipet region in the month of July 2014. Samples were collected in plastic containers previously thoroughly cleansed with distilled water and subsequently with sampled groundwater before filling. The fluoride concentration in groundwater was determined electrochemically, using Thermo Scientific Orion Star A214 Benchtop pH/ISE meter, using the USEP ion selective electrode method. As per experimental requirement, 2 ml of total ionic strength adjusting buffer grade III (TISAB III) was added in 20 ml of groundwater sample and determined the fluoride concentration. Calcium (Ca2+) and magnesium (Mg2+) were determined titrimetrically using standard EDTA method. Chloride (Cl−) was determined by standard AgNO3 titration. Carbonate (CO3 2−) and bicarbonate (HCO3 −) were determined by titration with HCl. Sodium (Na+) and potassium (K+) were measured by flame photometry. Sulfate (SO4 2−) and nitrate (NO3 −) were determined using UV–visible spectrophotometer. The EC and pH of water samples were measured in the field immediately after the collection of the samples using pH/EC/TDS meter (Hanna HI 9811-5). Sampling, preservation, and analysis of water samples were carried out following the method recommended by APHA (2005).
Hydrogeochemistry
Results of the hydrochemical parameters and corresponding groups of individual groundwater samples are presented in Table 1, and descriptive statistics for F− and other parameters are given in Table 2. Table 3 presents the correlation matrix in the analyzed groundwater samples of hard rock aquifers of Siddipet, Telangana State, India. Among the physical parameters, pH ranges from 6.3 to 8.9 with an average of 7.5, indicating the alkaline nature of groundwater. Even though pH has no effect on human health, it is closely related to other chemical constituents of water. According to Keshavarzi et al. (2010), in acidic water, fluoride is adsorbed on a clay surface, while in alkaline water, fluoride is desorbed from solid phases; therefore, alkaline pH is more favorable for fluoride dissolution, which is also observed by several other authors (Rafique et al. 2009; Saxena and Ahmed 2003; Rao 2009; Ravindra and Garg 2007; Vikas et al. 2009). In the present case, group III and IV water samples were found alkaline in nature and their pH value varies from 7.3 to 8.9. It is interesting that about 84 % of the samples lie between pH 7.1 and 8.9 (Tables 1, 2), which indicates that the dissolved carbonates are predominantly in the HCO3 − form (Adams et al. 2001). A positive correlation (Fig. 1a; Table 3) is seen between the pH of groundwater and the fluoride content indicating that the pH, and hence alkalinity, influences the fluoride content in the groundwater. In general, the concentration of nitrate does not exceed 10 mg L−1 in water under natural conditions (Cushing et al. 1973). However, nitrate varies from 9 to 361, 20 to 321, 22 to 194, and 9 to 198 mg L−1 in group I–IV, respectively (Table 1).
The possible sources of nitrates are poultry farms, animal wastages and septic tank leakages, and agricultural activities, which are noticed in the study area. These results suggest that groundwater has an elevated level of nitrate, greater than the drinking water guideline value of 45 mg L−1 (WHO 2011). The presence of high nitrate concentration in the drinking water increases the incidence of gastric cancer and other potential hazards to infants and pregnant women (Nagireddi Srinivasa Rao 2006) birth malformations, and hypertension (Majumdar and Gupta 2000). Chloride occurs naturally in all types of water. The concentration of chloride content in the water samples was recorded from 25 to 973, 28 to 746, 57 to 511, and 36 to 675 mg L−1 (Table 1). The majority of groundwater shows concentration of chloride above the WHO (2011) suggested maximum permissible limit of 250 mg L−1. The bicarbonate concentration in the groundwater ranges from 24 to 104, 31 to 134, 31 to 104, and 18 to 99 mg L−1 in group I–IV, respectively (Table 1). The high concentration of bicarbonate when compared to carbonate in the water is the result of the reactions of soil CO2 with dissolution of silicate minerals. TDS include inorganic salts, such as calcium, magnesium, potassium, and organic matter that are dissolved in water. As per the TDS classification (Fetters 1990) 72, 70, 60, and 40 % from group I, II, III, and IV belongs to brackish type (TDS >1000 mg L−1). Electrical conductivity ranges from 1070 to 3740, 1010 to 3850, 1040 to 3170, and 1260 to 1870 μS/cm from groups I, II, III, and IV, respectively (Table 1). According to a report of International Water Management Institute (IWMI), the TDS did not play direct role in health risks, but prolonged consumption of high salt containing water (TDS above 500 mg L−1) can cause kidney stone, a phenomenon widely reported from many parts of the country. The sodium concentration in groundwater ranges from 31 to 117, 17 to 121, 23 to 134, and 30 to 102 mg L−1, in group I–IV, respectively (Tables 1, 2). The high concentration of sodium ions among the cationic concentrations reflects rock weathering and/or dissolution of soil salts stored by the influence of evaporation (Stallard and Edmond 1983). The permissible limit of Na+, in potable water is 200 mg L−1, and none of the samples exceed the limit. The concentration of calcium ranges from 10 to 186, 26 to 144, 14 to 144, and 10 to 50 mg L−1 and magnesium 6 to 112, 15 to 87, 6 to 71 and 6 to 30 mg L−1, respectively (Table 1). The calcium and magnesium ions present in the groundwater are possibly derived from leaching of calcium and magnesium-bearing rock-forming silicates. The permissible limit for calcium and magnesium set by WHO are 200 and 150 mg L−1, respectively; samples from all the locations/groups have calcium and magnesium concentrations well below this limit (Table 1). The total hardness is varying from 65 to 565, 75 to 415, 60 to 330, and 50 to 225 mg L−1. Groundwater of the entire study area lies within the maximum permissible limit of 600 mg L−1 prescribed by WHO (2011). Sawyer and McCarthy (1967) classified groundwater, based on TH, as groundwater with TH <75, 75–150, 150–300 and >300 mg L−1, designated as soft, moderately hard, hard, and very hard, respectively. The analytical result indicates the water in the study area is moderately hard to very hard. The hardness of the water is due to the presence of alkaline earths such as calcium and magnesium. High concentration of TH in water may cause kidney stone and heart disease in human.
Discussion
To understand the chemical characteristics of groundwater in the hard rock aquifers of Siddipet, groundwater samples were plotted in Piper trilinear diagram (Piper 1944; Fig. 2). The groundwater has been classified into different hydrochemical types, and the concentration of fluoride is found high in sodium bicarbonate (Na–HCO3), calcium chloride (CaCl), sodium bicarbonate chloride (Na–HCO3–Cl), calcium bicarbonate chloride (Ca–HCO3–Cl), and waters. Groundwater in the sodium bicarbonate (Na–HCO3) type always has very high fluorine contents. It is also suggested that silicate weathering domination and rock–water interaction are the primary factors in increasing the major ion concentration in the groundwater. The high Na+ concentration in groundwater may be related to the cation exchange mechanism in the aquifers (Kangjoo Kim and Seong-Taekyun 2005). The deficiency of calcium ion concentration in the groundwater favors fluorite dissolution leading to excess of fluoride concentration (Table 1).
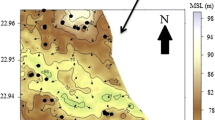
Gibbs diagram represents the ratio of Na+/(Na++Ca2+) and Cl−/(Cl−+HCO3 −) as a function of TDS, which is widely used to assess the functional sources of dissolved chemical constituents, such as precipitation dominance, rock dominance, and evaporation dominance (Gibbs 1970; Fig. 3a, b). The groundwater of groups III and IV are influenced largely by water–rock interaction when compared to the groundwater of groups I and II. The water–rock interaction and aquifer material played major role in evolution of water chemistry, which was further influenced by the evaporation process. Geological location is one of the most important factors affecting groundwater quality. The concentration of F− in the groundwater is found to increase with an increase in Na+ (Table 1). A significant positive correlation occurs between F− and lithogenic Na+ (Fig. 1b; Table 3). Thus, the lithogenic Na+ can be used as an index of weathering of minerals (Ramesam and Rajagopalan 1985). The weathering caused by alternative wet and dry conditions of the arid, and semi-arid climate is responsible for the leaching of F− from the minerals in the soils and rocks (Subba 2003; Wodeyar and Sreenivasan 1996; Subba et al. 1998a, b; Saxena and Ahmed 2001). Evaporation process is a common phenomenon in groundwater system. Evaporation will increase the concentration of total dissolved solids in groundwater, and the Na+/Cl− ratio remains the same, and it is one of the good indicative factors of evaporation (Fig. 1c). This observation indicates that evaporation may not be the major geochemical process controlling the chemistry of groundwater in this study region or ion exchange reaction dominating over evaporation. It is also observed that the ratio of Na+/Ca+ is increased with reference to the increase in fluoride concentration (Fig. 1d) in all groups. A negative correlation between TDS and F− is observed which suggests the influence of rock–water interaction (Fig. 1e; Table 3). Na+/Cl− ratio is found greater than one in all groups indicating that the Na is released from silicate weathering reactions (Meybeck 1987). Evidences for silicate weathering are shown by the relationships of Na+ vs HCO3 − and (Ca++Mg+) vs (SO4 2−+HCO3 −) (Fig. 4a, b). The F−-bearing water was found to have a negative relationship with Ca2+, which indicates that high F− in groundwater is associated with low Ca2+ content (Table 1; Fig. 5a); this is in agreement with the previous finding (Handa 1975). Fluorite, the main mineral that controls the geochemistry of F− in most environments is found in significant amount in granite, granite gneisses, and pegmatite (Deshmukh et al. 1995). F− concentration in study area ranges from 0.2 to 2.26 mg L−1 with a mean of 1.1 mg L−1 (Tables 1, 2). Concentration of F− marginally exceeds the permissible limit of drinking water (1.5 mg L−1) in about 22 % of the groundwater samples. The present investigation indicated that the high F− concentration more than 1.5 mg L−1 are observed in Nanchrpalli, Ponnala, Silanagar, Ganpur, Venkatapuram, Irkod, Rampur, Appannapalli, Pullur, Ankampet, Raghapuram, Randampalli, Narsapur, Mittapalli, Boggolonibanda, and Nancharpalli villages. Distribution of F− in groundwater of the Siddipet area is presented in Fig. 5b, and high F− concentration has been noticed in southern part of investigated area. Variations in the concentration of F− in groundwater from the study area suggest preferential dissolution of F− bearing minerals due to variation in the control parameters. Finally, bedrock containing fluorine minerals is responsible for the high F− in the groundwater of the study area.
Gibbs (1970) diagram illustrating the mechanisms controlling the chemistry of groundwater samples of the hard rock aquifer area, plot of Na/Na+Ca vs TDS and Cl/(Cl + HCO3) vs TDS
The presence of high F− (with low EC) zones in the investigated region, namely Boggulonibanda (2.2 mg L−1,1390 µS cm−1), Ponnala (2.1 mg L−1, 1690 µS cm−1), Silanagar (2 mg L−1, 1870 µS cm−1), Ganpur (1.7 mg L−1, 1040 µS cm−1), Irrkod (1.6 mg L−1, 1490 µS cm−1), Rampur (1.8 mg L−1, 1860 µS cm−1), Appannapalli (1.8 mg L−1, 1860 µS cm−1), Pullur (1.8 mg L−1, 1830 µS cm−1), and Ankampet (1.7 mg L−1, 1890 µS cm−1) suggests preferential dissolution of F− bearing minerals. The study area is predominantly occupied by granite/granitic gneiss. It is likely that these rocks could be providing higher F− to groundwater during weathering. Koritnig (1951) suggested that F− is leached in the initial stages of weathering of granite massifs. The weathering and leaching processes, mainly by moving and percolating water, play an important role in the incidence of F− in groundwater. The F− concentration in groundwater depends upon the following factors like climate, relief, evaporation, precipitation, geology, and geomorphology of the area. It is generally accepted that groundwater is enriched in F− due to prolonged water–rock interactions (Saxena and Ahmed 2001; Gizaw 1996; Frengstad et al. 2001; Carrillo-Rivera et al. 2002). The present study area represents granitic aquifers affected by very high amount of fluoride content with a maximum of 2.2 mg L−1. The problem is further aggravated due to lack of alternate source of drinking water, many a times the entire village depends upon a single source for cooking and drinking purposes. Therefore, it is suggested that the government authorities take serious steps to supply drinking water with low fluoride to the identified fluoride endemic villages in hard rock aquifers of Siddipet, Telangana State, South India.
Conclusion
Hydrogeochemical investigation carried out in the siddipet area of Telangana State revealed that the concentration of F− in groundwater is ranging from 0.2 to 2.2 mg L−1. 22 % of groundwater samples in the villages of Nanchrpalli, Ponnala, Silanagar, Ganpur, Venkatapuram, Irkod, Rampur, Appannapalli, Pullur, Ankampet, Raghapuram, Randampalli, Narsapur, Mittapalli, Boggolonibanda, and Nancharpalli exceed the drinking water standard of 1.5 mg L−1 set by WHO, which is responsible for the endemic dental fluorosis in these areas. The area is occupied by granitic/granitic genesis of the Archean age. The F−-bearing minerals apatite, muscovite, and biotite are present in these rocks are responsible for the higher concentration of F− in the groundwater due to rock–water interaction.
References
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
Apambire WB, Boyle DR, Michel FA (1997) Geochemistry, genesis, and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environ Geol 33:13–24
APHA (2005) Standard methods for examination of water and wastewater, 21st edn. American Public Health Association, American Water Works Association and the Water and Environment Federation, Washington
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487
Carrillo-Rivera JJ, Cardona A, Edmunds WM (2002) Use of abstraction regime and knowledge of hydrogeological conditions to control high-fluoride concentration in abstracted groundwater: San Luis Potosı basin, Mexico. J Hydrol 261:24–47
Cushing EM, Kantrowitz IH, Taylor KR (1973) Water resources of the Delmarva Peninsular. U S geological survey professional paper 822, Washington DC
Deshmukh AN, Shah KC, Sriram A (1995) Coal Ash: a source of fluoride pollution, a case study of Koradi thermal power station, District Nagpur, Maharashtra. Gondwana Geol Mag 9:21–29
Fantong WY, Satake H, Ayonghe SN, Suh EC et al (2009) Geochemical provenance and spatial distribution of fluoride in groundwater of Mayo Tsanaga river basin, Far North Region, Cameroon: implications for incidence of fluorosis and optimal consumption dose. Environ Geochem Health 32:147–163
Felsenfeld AJ, Robert MA (1991) A report of fluorosisin the United Statessecondary to drinking well water. J Am Med Assoc 265(4):486–488
Fetter CW (1990) Applied hydrogeology. CBS Publishers and Distributors, New Delhi
Fordyce FM, Vrana K, Zhovinsky E, Povoroznuk V, Toth G, Hope BC, Iljinsky U, Baker J (2007) A health risk assessment for fluoride in Central Europe. Environ Geochem Health 29:83–102
Frengstad B, Banks D, Siewers U (2001) The chemistry of Norwegian groundwaters: the dependence of element concentrations in crystalline bedrock groundwaters. Sci Total Environ 277:101–117
Gaciri SJ, Ad Davis TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412
Ghosh Aniruddha, Mukherjee Kakali, Sumanta KG, Saha Bidyut (2013) Sources and toxicity of fluoride in the environment. Res Chem Intermed 39:2881–2915
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090
Gizaw B (1996) The origin of high bicarbonate and fluoride concentrations in waters of the main Ethiopian Rift Valley. J Afr Earth Sci 22:391–402
Gupta S, Banerjee S, Saha R, Datta JK, Mondal N (2006) Fluoride geochemistry of ground water in Nalhati-1 block of Birbhum district, West Bengal, India. Fluoride 39(4):318–320
Handa BK (1975) Geochemistry and genesis of fluoride containing groundwaters in India. Groundwater 13:275–281
Hong-jian Gao, You-qian Jin, Jun-ling Wei (2013) Health risk assessment of fluoride in drinking water from Anhui Province in China. Environ Monit Assess 185:3687–3695
Indian Council of Medical Research (ICMR) (1975) Manual of standards of quality for drinking water supplies. In: Special report series, 2nd edn. New Delhi, p 44
Jacks G, Rajagopalan K, Alveteg T, Jonsson M (1993) Genesis of high-F groundwaters, Southern India. Appl Geochem 2:241–244
Jha SK, Nayak AK, Sharma YK (2010) Potential fluoride contamination in the drinking water of Marks Nagar, Unnao district, Uttar Pradesh, India. Environ Geochem Health 32:217–226
Kaminsky LS, Mahoney MC, Leach J, Melius J, Miller JM (1990) Fluoride: benefits and risks of exposure. Crit Rev Oral Biol Med 1:261–281
Karro Enn, Uppin Marge (2013) The occurrence and hydrochemistry of fluoride and boron in carbonate aquifer system, central and western Estonia. Environ Monit Assess 185:3735–3748
Karthikeyan G, Anitha CED, Vishwanathan G (2005) Effect of certain macro and micro minerals on fluoride toxicity. Indian J Environ Prot 25:601–609
Keshavarzi B, Moore F, Esmaeili A, Rastmanesh F (2010) The source of fluoride toxicity in Muteh area, Isfahan, Iran. Environ Earth Sci 61:777–786
Kim Kangjoo, yun Seong-Taek (2005) Buffering of sodium concentration by cation exchange in the groundwater system of a sandy aquifer. Geochem J 39:273–284
Koritnig S (1951) Ein Beitrag zur Geochemie des Fluor (A contribution to the geochemisty of fluorine). Geochim Cosmochim Acta 1:89–116
Li Y, Liang CK, Katz BP, Niu S, Cao S, Stookey GK (1996) Effect of fluoride exposure and nutrition on skeletal fluorosis. J Dent Res 75:2699
Majumdar D, Gupta N (2000) Nitrate pollution of ground water and associated human health disorders. Indian J Environ Health 42(1):28–39
Mesdaghinia Alireza, Vaghefi Kooshiar Azam, Montazeri Ahmad, Mohebbi Mohammad Reza, Saeedi Reza (2010) Monitoring of fluoride in groundwater resources of Iran. Bull Environ Contam Toxicol 84:432–437
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Moghaddam Asghar Asghari, Fijani Elham (2008) Distribution of fluoride in groundwater of Maku area, northwest of Iran. Environ Geol 2008:56–116
Murray JJ (1996) Appropriate use of fluorides for human health. World Health Organization, Geneva
Narsimha A, Sudarshan V (2013) Hydrogeochemistry of groundwater in Basara area, Adilabad District, Andhra Pradesh, India. J Appl Geochem 15(2):224–237
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar desert, Pakistan. Chemosphere 78:1313–1321
Oruc N (2008) Occurrence and problems of high fluoride waters in Turkey: an overview. Environ Geochem Health 30:315–323
Ozsvath DL (2006) Fluoride concentrations in a crystalline bedrock aquifer Marathon County. Environ Geol 50:132–138
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–923
Rafique T, Naseem S, Usmani TH, Bashir E, Khan FA, Bhanger MI (2009) Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J Hazard Mater 171:424–430
Ramesam V, Rajagopalan K (1985) Fluoride ingestion into the natural water of hardrock areas, peninsular India. J Geol Soc India 26:125–132
Rao Nagireddi Srinivasa (2006) Nitrate pollution and its distribution in the groundwater of Srikakulam district, Andhra Pradesh, India. Environ Geol 51(4):631–645
Rao NS (2009) Fluoride in groundwater, Varaha river basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 152:47–60
Ravindra K, Garg VK (2007) Hydro-chemical survey of groundwater of Hisar City and assessment of defluoridation methods used in India. Environ Monit Assess 132:33–43
Sawyer GN, McCarthy DL (1967) Chemistry of sanitary engineers, 2nd edn. Mc Graw Hill, New York, p 518
Saxena VK, Ahmed S (2001) Dissolution of fluoride in groundwater: a water–rock interaction study. Environ Geol 40:1084–1087
Saxena V, Ahmed S (2003) Inferring chemical parameters for the dissolution offluoride in groundwater. Environ Geology 43(6):731–736
Short HE, McRobert TW, Bernard AS, Mannadinayer AS (1937) Endemic fluorosis in Madras presidency. Indian J Med Res 25:553–561
Stallard RE, Edmond JM (1983) Geochemistry of Amazon River: the influence of the geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Subarayan BG, Viswanathan Gopalan, Siva IS (2012) Prevalence of fluorosis and identification of fluoride endemic areas in Manur block of Tirunelveli district, Tamil Nadu, South India. Appl Water Sci 2:235–243
Subba RN (2003) Groundwater quality: focus on fluoride concentration in rural parts of Guntur district, Andhra Pradesh, India. Hydrol Sci J 48:835–847
Subba Rao N, Krishna Rao G, John Devadas D (1998a) Variation of fluoride in groundwaters of crystalline terrain. J Environ Hydrol 6:1–5
Subba Rao N, Prakasa Rao J, Nagamalleswara Rao B, Niranjan Babu P, Madhusudhana Reddy P, John Devadas D (1998b) A preliminary report on fluoride content in groundwaters of Guntur area, Andhra Pradesh, India. Curr Sci 75:887–888
Susheela AK, Kumar A, Bhatnagar M, Bahadur R (1993) Prevalence of endemic fluorosis with gastro-intestinal manifestations in people living in some North-Indian villages. Fluoride 26:97–104
Vikas C, Kushwaha K, Pandit MK (2009) Hydrochemical status of groundwater in district Ajmer (NW India) with reference to fluoride distribution. J Geol Soc India 73:773–784
WHO (2011) Guidelines for drinking-water quality, 4th edn, vol 1: recommendations. World Health Organization, Geneva
Wodeyar BK, Sreenivasan G (1996) Occurrence of fluoride in the groundwaters and its impact in Peddavankahalla basin, Bellary district, Karnataka, India—a preliminary study. Curr Sci 70:71–74
Acknowledgments
The authors sincerely thank the anonymous reviewers for their very keen observations and helpful suggestions which had enhanced the quality of the paper. Financial support of the A. Narsimha by the Department of Science and Technology (DST)—Science and Engineering Research Board (SERB) Government of India, New Delhi under the Start-Up Research Grant (Young Scientists) project (SR/FTP/ES-13/2013) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Narsimha, A., Sudarshan, V. Contamination of fluoride in groundwater and its effect on human health: a case study in hard rock aquifers of Siddipet, Telangana State, India. Appl Water Sci 7, 2501–2512 (2017). https://doi.org/10.1007/s13201-016-0441-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0441-0