Abstract
Intercropping of legumes and cereals has many benefits to both plant partners. In this study, the effect of legume-maize intercropping on plant growth, grain yield and N2 fixation of six legumes was assessed using the 15N natural abundance and ureide techniques. For this, a field experiment involving six legume species and two cropping systems was established at the Malkerns Research Station, Eswatini during the 2017/2018 cropping season. Based on the 15N isotopic and ureide analysis, the six test legumes respectively obtained 39.06 – 70.19% and 16.46 – 55.79% of their N nutrition from symbiosis. The amounts of N-fixed ranged from 12.66 to 66.57 kg ha−1. In general, high amount of N-fixed by legumes correlated strongly with greater shoot dry matter accumulation (r = 0.7981; p < 0.001) and high grain yield (r = 0.5905; p < 0.001), indicating the importance of N2 fixation in plant growth and reproduction. Legumes grown under monocropping recorded higher plant growth, symbiotic performance and grain yield when compared to those grown in mixed culture with maize. However, shoot %Ndfa was much higher under intercropping than sole cropping due to competition by cereal and legume for soil N. Components of maize yield were similar for the two cropping systems. The %N derived from fixation and %relative ureide-N abundance were significantly correlated (r = 0.4005; p < 0.001), indicating that the 15N natural abundance technique and the ureide method were complementary in measuring N2 fixation in the test legumes. These results have provided some insights on the impact of cropping system on plant growth, symbiotic performance and grain yield of six selected legumes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cereal-legume intercropping plays a central role in smallholder farming in developing countries, especially where water resources are limited (Tsubo et al. 2005). In Southern Africa, smallholder farmers generally grow drybean as an intercrop with maize, the main staple crop. When associated with soil bacteria called rhizobia, legumes form root nodules, and the bacteria inside these structures are able to reduce atmospheric N2 into NH3 for their own use. Although, the excess NH3 is available for plant use, it can also be excreted from the nodules into the soil for uptake by associated plants (Dahmardeh et al. 2010). One of the earliest studies found that an intercropped legume could contribute as much as 40 kg N ha−1 to an associated maize crop (Willey 1979). Legumes are therefore a sustainable source of N in cereal-legume intercropping systems (Shen and Chu 2004). Several studies have shown that legume-maize intercropping can produce greater yields than either species grown alone (Li et al. 1999; Dahmardeh et al. 2010; Manasa 2020; Nasar et al. 2020).
The inclusion of legumes in cropping systems also has the advantage of making extra N available to subsequent cereal crops from biological N2 fixation (Ghanbari‐Bonjar 2003; Dahmardeh et al. 2010). Furthermore, legumes can directly benefit associated cereal crops in low-N soils through N transfer from nodules to the cereal (Shen and Chu 2004), thus decreasing the N fertilizer requirements of cereals in an intercrop (Shen and Chu 2004). Additionally, intercropping is known to achieve higher grain yield than monoculture through improving the efficient use of water, light, nutrients, and other resources (Li et al. 2011). In fact, most intercropped crop species are complementary, as they generally use a given resource differently based on time, space, and growth characteristics (Zhang et al. 2014). Maitra et al. (2020) also found that success in a maize-legume intercropping system was largely dependent on factors such as the choice of crops, their maturity, density, and time of planting.
Currently, there are several methodologies for quantifying N2 fixation by nodulated legumes and these include the N balance, N difference, 15N isotope dilution, ureides concentration and acetylene reduction techniques (Peoples et al. 1989; Herridge and Giller 2016). Nitrogen balance compares the total N of a plant-soil system on two separate occasions, with any increase attributed to N2 fixation after other possible inputs and outputs of N have been accounted for. On the other hand, the N difference method compares total N accumulated by the N2-fixing plants with that of neighbouring non N2-fixing plants, with the difference assumed to be due to N2 fixation (Unkovich et al. 2008). The 15N and ureide methods provide estimates of the percentage of total N of the legume crop that is derived from N2 fixation (%Ndfa) (Herridge and Giller 2016) whereas the acetylene reduction technique assays the activity of nitrogenase, the enzyme catalyzing N2 fixation (Peoples et al. 1989:2; Herridge and Giller 2016).
In the current study, the 15N natural abundance and ureide techniques were employed to quantify the biological nitrogen fixation of selected test legumes. Despite the many benefits of intercropping, little information currently exists on maize-legume mixed culture in Eswatini, where maize intercropping is dominant. The aim of this study was to evaluate the effect of maize and legume intercropping on plant growth, symbiotic performance, and grain yield of six selected grain legumes in Eswatini.
2 Materials and methods
2.1 Study site
A field experiment was conducted at the Malkerns Research Station (26°33’S, 31°10’E), located in the Middleveld Agro-ecological zone of Eswatini. The area has a mean annual rainfall ranging from 800 to 1000 mm and an annual mean temperature of 7 °C to 26.6 °C (Edje and Ossom 2009; Dlamini et al. 2021). The daily temperatures recorded during the cropping season (December to May) ranged from 12.1 °C to 18.3 °C for minimum and 25.3 °C to 28.9 °C for maximum in the 2017/2018 cropping season (Table 1). The total rainfall recorded during the study period was 2986 mm in 2017, and 1189.2 mm in 2018. However, the total rainfall from planting to harvest was 816.4 mm during the 2017/2018 cropping season. The soil type at the Malkerns Research Station is classified as Deep Red Loam using the Malkerns series (Kunene et al. 2019). Prior to planting, soil samples were collected from plots across the field, and bulked for analysis of soil chemical and physical properties. The bulk soil had pH 5.15 and contained 19.63 cmol( +) kg−1 CEC, 1.14% SOC, 0.075% N, 14 mg kg−1 P, 54 mg kg−1 K, 0.91 mg kg−1 Cu, 461 mg kg−1 Ca, 204 mg kg−1 Mg, 0.91 mg kg−1 Co, 452.80 mg kg−1 Fe and 1.69 mg kg−1 Zn.
2.2 Experimental design and planting
The field experiment involved intercropping maize with six selected legumes. The maize and legumes were planted as sole crops or intercropped in a randomised complete block design, with 13 treatments, and replicated four times. The treatments consisted of maize (cv. SC 403) and six legumes [namely, Bambara groundnut, cowpea (Mtilane local), drybean (cv. Kranskop), groundnut (cv. Natal common), jack bean (Accession 493) and soybean (Nukwa local)] planted as sole crops or intercropped with maize (Supplementary Table S1). Nitrogen was applied to the field as a blanket treatment at a single rate of 10 kg N ha−1 using NPK (2:3:2). The experimental plots measured 4.5 m by 5 m (22.5 m2) with 1 m separating adjacent plots and blocks. For sole maize, two seeds were planted per hole at a depth of 5 cm and later thinned to one seedling at a spacing of 0.9 m by 0.25 m. For sole-planted legumes, two seeds were sown per hole at 5 cm depth and thinned to one plant with 0.9 m by 0.15 m spacing. With intercropping, Bambara groundnut, cowpea, drybean, groundnut, jack bean and soybean seeds were planted in between two rows of maize with 45 cm spacing from the maize plants. Weeds were controlled manually using hand hoe, and pests controlled chemically using a mixture of Chlorpyrifos and Beef oil. Ploughing of the experimental field was done mechanically and harrowing done with a disc plough. Bulk soil samples were collected across the field at a depth of 30 cm, pooled and air-dried at room temperature, sieved (2.0 mm sieve) and analyzed at the Agricultural Research Council, Pretoria and at the Department of Agriculture, Elsenburg, Western Cape, for pH, soil organic carbon, cation exchange capacity and mineral nutrients.
2.3 Sampling for nodulation and plant biomass
Four legume plants per plot were sampled at early podding stage by digging them up and separating into shoots and nodulated roots. The shoots were stored in labelled brown paper bags and the roots with intact nodules were kept in plastic Ziploc bags, and transported to the laboratory. The roots were then gently washed to remove debris, and the nodules detached, counted and stored in silica gel prior to bacterial isolation and use in bacterial diversity studies. The shoot samples were each oven-dried at 65 °C to a constant weight to determine shoot dry matter yield. The shoots were then finely ground (0.85 mm) for isotopic analysis to assess N2 fixation of the selected test legumes. The stems plus petioles of Bambara groundnut, cowpea, drybean, jack bean and soybean, which belong to the tribe Phaseoleae, were weighed and ground for ureide analysis as an additional measure of N2 fixation.
To measure soil N uptake by the legumes, non-legume plant species growing within the experimental plots were sampled concurrently as the test legumes, and the shoots processed for 15N/14N isotopic analysis. These non-legume plants were used as reference plants to measure soil N uptake by the test legumes.
2.4 Shoot 15 N/ 14 N analysis
15N isotopic analysis was performed at the Stable Light Isotope Laboratory, University of Cape Town, South Africa. Briefly, 2.5 mg of ground legume samples or reference plant samples were weighed into tin capsules and fed onto a Carlo Erba NA1500 Elemental Analyzer coupled to a Finnigan MAT 252 Mass Spectrometer (Finnigan MAT GmbH, Bremen, Germany) via a Conflo II Open-Split Device. An internal standard of Nasturtium spp. was included after every five runs of the plant samples to correct for machine error associated with the isotopic analysis. The δ15N values of each of the six test legumes were calculated using the equation (Unkovich et al. 2008):
where 15N/14Nsample is the abundance ratio of 15N and 14N in the plant sample, and 15N/14Natm is the abundance ratio of 15N and 14N in atmospheric air.
The %N in shoot was obtained directly from the mass spectrometer, and the shoot N content calculated as the product of %N and shoot dry matter.
The proportion of legume N derived from atmospheric N2 fixation (%Ndfa) was calculated as (Unkovich et al. 2008):
where, δ15Nref is the 15N natural abundance of the reference plant, δ15Nleg is the 15N natural abundance of the legume, and the B-value is the 15N natural abundance of the test legume solely dependent on N2 fixation for its N nutrition.
2.5 Determination of B-value
The B values used for Bambara groundnut, cowpea, drybean, groundnut and soybean were obtained from literature as -1.40, -1.61, -2.16, -0.88 and -1.83‰ respectively (Unkovich et al. 2008; Mohale et al. 2014). However, the B value of jack bean used in this study was experimentally determined in the glasshouse. For this, seeds of jack bean were surfaced-sterilised in 95% ethanol, followed by soaking in 3% sodium hypochlorite solution. The jack bean seeds were then rinsed five times in sterile distilled water and planted in autoclaved sand contained in plastic pots with sterile non-absorbent cotton covering the top to avoid contamination. Three pots were used for each of the isolates. The germinated seeds were inoculated with 1 mL broth suspension of rhizobial isolates earlier obtained from the root nodules of the jack bean. The plants were watered with N-free nutrient solution (Broughton and Dilworth 1971).
Sixty days after planting, the seedlings were harvested and separated into shoots, roots and nodules. The shoots and roots were oven-dried (65 °C) for 48 h and weighed. Shoots and roots of each plant were separately ground and analyzed for 15N/14N isotopes using mass spectrometry. The mean δ15N value (-0.77‰) was calculated and used as the B-value to estimate the proportion of N derived from atmospheric N2 fixation by jack bean..
2.6 Amount of N-fixed in shoots
The N-fixed by each test legume species was calculated as:
2.7 Soil N uptake
Soil N uptake was determined as the difference between legume total N and the amount of N-fixed.
2.8 Analysis of ureides and nitrate in stems and petioles
2.8.1 Solute extraction from ground stems and petioles
A 0.5 g ground stem plus petiole sample was weighed and transferred into 100 mL Erlenmeyer flask, and 25 mL distilled water added to each sample and boiled for 2 min in a hot water bath. The hot samples were filtered into a 50 mL Erlenmeyer flask using 15 cm Whatman No. 40 filter paper. The residue left was washed with distilled water, allowed to cool and the volume brought to 50 mL with distilled water. The extract was then stored at -20 °C in small vials prior to analysis of N solutes. Ureide and nitrate analyses were performed as described by Unkovich et al. (2008).
2.8.2 Ureide analysis
The concentration of ureides in plant extracts was determined colorimetrically as described by Young and Conway (1942). A standard curve of optical density (OD) plotted against the concentrations of allantoin standards (Fig. 1A) was used to extrapolate the concentration of ureide in the test sample. Briefly, 0.5 mL volume of the plant extract was pipetted into duplicate test tubes and made up to 2.5 mL with distilled water. Also, 2.5 mL of control (water) blanks and 2.5 mL of each of the five ureide standards (0, 0.01, 0.02, 0.04 and 0.10 mM) were included and 0.5 mL of 0.5 M NaOH added to each extract and standard. The test tubes with samples were placed in boiling water for 10 min, and removed from the water bath to cool on the bench at room temperature. Thereafter, 1.0 mL HCl / phenyl hydrazine chloride solution (100 mL 0.65 M HCl + 0.33 g phenyl hydrazine in 100 mL distilled water) was added to each tube and vortexed. The samples were then boiled for 2 min in a hot water bath and immediately placed in ice for 15 min, followed by adding 2.5 mL 10 M HCl / potassium ferricyanide solution and mixing thoroughly for colour to develop. Absorbance readings were taken after 10 min of colour development. The optical density (OD) was read at 525 nm on a UV–Visible Spectrophotometer (JENWAY 7300, Bibby Scientific Ltd, Stone, Staffs). The equation from the standard curve generated (Fig. 1A) was used to compute the concentration of ureides (mM) in the plant extracts and expressed as µg ureide-N. mL−1 of plant extract.
2.8.3 Nitrate assay
Nitrate–N in the plant extracts was analyzed using the salicylic acid method (Cataldo et al. 1975), as described by Unkovich et al. (2008). A standard curve was constructed of optical density versus known nitrate concentrations (Fig. 1B), and the absorbances of extracts read from the standard curve. Duplicate tubes were used for each nitrate concentration and the control (water). Briefly, 0.05 mL of each extract, standard or water (control) was pipetted into duplicate test tubes, and 0.20 mL salicylic/sulphuric acid added, vortexed and left to stand on the bench at room temperature for 20 min, then 4.75 mL 2 M NaOH was added, and left to stand on the bench at room temperature for 10 min for a yellow colour to develop. The optical density was quickly read at 410 nm using the UV–Visible Spectrophotometer (JENWAY 7300, Bibby Scientific Ltd, Stone, Staffs). The nitrate concentration in each test sample was calculated using the equation from the standard curve (Fig. 1B). The nitrate concentration in the samples was expressed as µg nitrate–N. mL−1 of plant extract.
Percent relative ureide-N abundance.
The percent relative ureide-N abundance (RU–N) was calculated as:
where a and b are the molar concentrations of ureide-N (ureides contain four N atoms per molecule) and nitrate–N, respectively (Unkovich et al. 2008).
2.8.4 Grain yield determination
At physiological maturity, two inner rows per plot were harvested for both maize and each legume crop (Bambara groundnut, cowpea, drybean, groundnut, jack bean and soybean) to determine the grain yield. The pods were separated from the plants, air-dried to 15% moisture content and shelled to obtain the seeds. The maize cobs were air-dried to 12.5% moisture content and five cobs per plot shelled manually. The seeds were then weighed, and grain yield expressed per hectare based on the plant population. Additionally, 100-seed weight was determined for each test plant species.
2.9 Statistical analysis
The data collected were tested for normality before being subjected to a 2-way analysis of variance using Statistica (version 10.1). Duncan’s multiple range test was used to separate means that showed significant differences at p ≤ 0.05. Correlation analyses were performed to assess the relationship between measured parameters.
3 Results
3.1 Shoot δ 15 N values of reference plants
The combined mean δ15N value of all the reference plants collected from the experimental plots was used to calculate soil N uptake (+ 7.79‰) by the six legumes (Supplementary Table S2).
3.2 Plant growth, symbiotic parameters and grain yield
3.2.1 Main effect of species
Of the six legumes tested, jack bean produced much larger amount of shoot DM, followed by soybean, groundnut, and then cowpea (Table 2), while drybean recorded the lowest shoot biomass. Shoot %N was however higher in soybean, followed by jack bean, and lowest in drybean (Table 2). Shoot N content was also much higher in jack bean, followed by soybean, and lowest in drybean. Shoot δ15N values were lowest in Bambara groundnut, and much higher in soybean (Table 2). As a result, percent N derived from fixation was highest in Bambara groundnut, followed by groundnut, and lowest in soybean. However, the amount of N-fixed was much greater in jack bean due to its bigger shoot DM, and lowest in drybean which recorded the least shoot biomass. Soil N uptake ranged from 13.4 to 90.7 kg ha−1, and was highest in jack bean. Grain yield was highest in jack bean, followed by Bambara groundnut and groundnut, and lowest in cowpea (Table 2).
3.3 Main effect of cropping system
Shoot biomass, N content, δ15N, amount of N-fixed and soil N uptake were all significantly higher in monocropping compared to intercropping (Table 2). Percent N derived from fixation was however much greater in mixed culture relative to monocropping. But shoot N concentration and grain yield were similar for sole and mixed culture (Table 2).
3.4 Species x cropping system interaction
Species x cropping system interaction was significant for shoot DM, shoot %N, N content, δ15N, percent N derived from fixation, amount of N-fixed, soil N uptake and grain yield (Table 2). Shoot %N was similar for sole and mixed cropping in Bambara groundnut, cowpea, drybean, groundnut and jack bean, but greater in monocultured soybean than mixed culture (Fig. 2A). Shoot N content was however higher under monocropping than intercropping for cowpea, drybean, groundnut, jack bean and soybean, but lower in sole crop relative to mixed cropping for Bambara groundnut (Fig. 2B). Shoot δ15N values were greater in monocultured Bambara groundnut, cowpea, drybean and groundnut than mixed culture, except for jack bean which recorded similar values in the two cropping systems and soybean which had the highest shoot δ15N in mixed culture (Fig. 2C). As a result of the higher shoot δ15N values under monocropping, the percent N derived from fixation was significantly lower in the shoots of all test species under sole cropping, except for soybean which derived more N from fixation in monoculture than mixed culture (Fig. 2C). The amount of N-fixed was much higher in sole cropped cowpea, groundnut and soybean than mixed culture. In contrast, N-fixed was greater in mixed cultured Bambara groundnut, and similar for drybean and jack bean in the two cropping systems (Fig. 2E). Soil N uptake was significantly increased in shoots of monocultured cowpea, drybean, groundnut, jack bean and soybean compared to the mixed culture, but similar for the two cropping systems in Bambara groundnut (Fig. 2F). Grain yield was generally higher in all six test species grown under monoculture, but significant for only Bambara groundnut, groundnut, jack bean and soybean (Fig. 3).
Interaction effect of species x cropping system on A) %N, B) N content, C) δ15N, D) Ndfa, E) N-fixed and F) soil N uptake of six legumes planted at Malkerns Research Station, Eswatini during the 2017/18 cropping season. In each species, bars with dissimilar letters are significantly different at p ≤ 0.05 and the error bars represents standard error (SE)
3.5 Growth and yield components of maize intercropped with legumes
Maize intercropped with Bambara groundnut recorded the highest shoot DM, followed by sole maize and maize intercropped with groundnut (Table 3). Cob length per plant, seed rows per cob, cob diameter, seed dry weight per plant, 100-seed weight and grain yield were similar for both sole and intercropped maize (Table 3).
3.6 Tissue ureide and nitrate concentrations
In the ureide analysis, the absorbance of samples were higher for soybean and cowpea when compared to the other legumes, followed by drybean, and lowest in jack bean. As a result, ureide concentration in stem + petiole was also markedly greater in soybean and cowpea and lowest in jack bean (Table 4). The optical density of nitrate in stem and petiole extracts was highest in soybean and lowest in Bambara groundnut (Table 4). As a result, nitrate levels were also much greater in soybean, and lowest in Bambara groundnut. Percent relative ureide-N abundance was highest in Bambara groundnut, cowpea and drybean, and lowest in jack bean (Table 4).
3.7 Main effect of cropping system
Optical densities of stem + petiole extracts, ureide and nitrate concentrations, as well as percent relative ureide-N abundance were all unaffected by cropping system (Table 4).
3.8 Species x cropping system interaction
Species x cropping system interaction was significant for ureide concentrations, nitrate levels in stem and petioles, as well as for the percent relative ureide-N abundance (Table 4). An analysis of species x cropping system interaction revealed marked differences in species response to the cropping systems (Fig. 4). As shown in Fig. 4A-C, Bambara groundnut and cowpea recorded significantly greater absorbances and ureide concentrations with intercropping relative to monoculture, while soybean revealed the reverse. The same parameters in drybean and jack bean were however unaffected by cropping system (Fig. 4A-C). Intercropping increased tissue nitrate absorbances and concentrations in Bambara groundnut, and decreased it in monoculture, but these parameters were unaffected by cropping system in cowpea, jack bean and soybean (Fig. 4D-F). Except for drybean, which showed decreased percent relative ureide-N abundance with intercropping, this parameter was unaffected by cropping system in Bambara groundnut, cowpea, jack bean and soybean (Fig. 4G).
Interaction effect of species x cropping system on A-C) Ureide concentration, D-F) Nitrate concentration and E) RU–N of six legumes planted at Malkerns Research Station, Eswatini during the 2017/18 cropping season. In each species, bars with dissimilar letters are significantly different at p ≤ 0.05 and the error bars represents standard error (SE)
4 Discussion
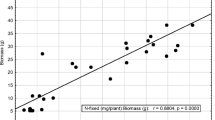
The ability of bacteroids in the root nodules of legumes to reduce atmospheric N2 to NH3 has huge nutritional benefits for associated intercropped partners, as well as to following cereal crops in rotation (Shen and Chu 2004; Mafongoya et al. 2006; Dahmardeh et al. 2010; Rusinamhodzi et al. 2012). In this study, differences in plant growth, symbiotic functioning, and grain yield were evaluated in six grain legumes intercropped with maize at Malkerns Research Station, Eswatini. The results revealed significant differences in plant growth, symbiotic performance and grain yield due to species differences, phenology and growth characteristics. Whereas the levels of key elements such as N (0.075%), P (14 mg kg−1) and K (54 mg kg−1) were generally low in the test soil, the plants in this study benefited from the blanket application of NPK fertilizer to plots prior to planting. In most instances, high shoot dry matter (SDM) in the test legumes was associated with high shoot δ15N, %N and N content as evidenced by jack bean which had the highest SDM, a relatively high shoot δ15N and %N as well as the highest N content (Table 2). Conversely, drybean had the lowest SDM, low δ15N and the lowest shoot %N and N content (Table 2). These findings were supported by the significant positive correlations found between shoot DM and %N (r = 0.5280), shoot DM and N content (r = 0.9582) (Fig. 5A, B). Furthermore, intercropping with maize caused low legume SDM from competition with maize and low δ15N from increased N2 fixation triggered by competition from the associated cereal plant for soil N. Monocultured legumes however had high SDM, δ15N and N content, an indication of reduced competition for water and mineral resources. The observed low shoot DM and low shoot δ15N values associated with intercropping in this study are consistent with the findings of Kermah et al. (2018). It has been shown that low shoot δ15N is generally the result of greater %Ndfa legumes (Mokgehle et al. 2014; Beyan et al. 2018; Lengwati et al. 2020). This was also the case in this study, with legumes that recorded low δ15N values exhibiting high %Ndfa, and vice versa. Bambara groundnut, for example, recorded the lowest shoot δ15N and the highest %Ndfa, while soybean which had the highest shoot δ15N recorded the lowest %Ndfa (Table 2). Although Kermah et al. (2018) found that the %Ndfa of legumes is not influenced by the cropping system but differs among legume species and study sites, this was not the case in this study as the cropping system had a marked effect on the %Ndfa of the test legumes (Table 2). Similar observations were made by Giller et al. (1991) who also found that intercropping of grain legumes with cereals generally resulted in the legume deriving a greater proportion of its N from symbiotic fixation than when grown in monoculture.
Due to their higher shoot biomass, the monocultured legumes produced more symbiotic N than their intercropped counterparts (Table 2). These results were similar to those of Giller (2001) and Adeleke and Haruna (2012), who also reported greater shoot DM with monoculture leading to increased amounts of fixed-N when compared to the mixed culture. Egbe et al. (2013) as well as Kermah et al. (2018) also found high amounts of N-fixed in Bambara groundnut, cowpea, groundnut and soybean monocultures than when intercropped with maize. In contrast, shoot percent N derived from N2 fixation was much higher under intercropping than monocropping due to intense competition for soil N by two partners in the former system. As a result, soil N uptake by legumes was lower under mixed culture than monoculture. Independent of the cropping system, the amount of N-fixed was significantly higher in jack bean, possibly due to its huge shoot biomass, which required more N for growth and development. In general, the amount of N-fixed and soil N uptake were higher in monoculture than mixed culture. As a result, grain yield was also greater under monocropping than intercropping. At species level, jack bean produced more grain, followed by Bambara groundnut and groundnut. In general, monocultures of the six legumes produced more grain yield than mixed cultures (Table 2).
In Southern Africa, smallholder farmers generally practice maize-legume intercropping in order to reduce total crop failure under monoculture (Rusinamhodzi et al. 2012; Kermah et al. 2017). In this study however maize yields were similar for both sole cropping and mixed culture (Table 3). These results contradict an earlier report by Hassan et al. (2014) on maize yield components under intercropping. Generally, maize grain yield has been reported to be higher under legume-maize intercropping than sole maize (Mthembu et al. 2018). However, Alhassan and Egbe (2014) recorded higher maize grain yield in sole maize relative to intercropped maize, and in this study there were no differences in grain yield under monocropping and intercropping. These inconsistences can be attributed to a number of factors including the plant heights of the legume and maize, sequential planting dates of legume and maize, level of shadiness caused by the cereal, the rhizosphere dynamics of the intercropped partner, etc. More field experiments are needed between short stalk maize and legumes (less shadiness), as well as long stalk maize and legumes (more shadiness), in order to resolve the yield benefits of intercropping on the two partners.
5 N2 fixation in five Phaseoleae legume species measured using the ureide assay vs. the 15N natural abundance technique
In this study, the ureide and 15N isotopic techniques were used to measure N2 fixation in the five ureide-exporting test legumes, and the results showed that the legumes with high ureide-N concentrations generally recorded low tissue nitrate–N concentrations, and vice versa, albeit a few exceptions (Table 4). Jack bean, for example, showed low ureide-N concentration and high tissue nitrate–N, while cowpea in contrast revealed high ureide and low nitrate–N levels (Table 4). High nitrate concentrations in the stem and petiole extracts of the test legumes in this study suggested their greater reliance on soil N uptake than symbiosis for their N nutrition (Herridge and Peoples 1990), while the recorded high ureide concentrations in stem extracts indicate greater N2 fixation (Hayat et al. 2008; Mohammed et al. 2022).
Consistent with the 15N natural abundance data, Bambara groundnut, cowpea and drybean also recorded the highest percent relative ureide-N abundance (55.79, 55.08 and 52.60%, respectively), while soybean (40.47%) and jack bean (16.46%) revealed the lowest %RU–N in conformity with the %Ndfa values from 15N isotopic analysis. Based on the two methods, Bambara groundnut, cowpea and drybean derived most of their N nutrition from N2 fixation, while jack bean and soybean obtained most of their N from uptake in the soil (Table 2 and 4). Hayat et al. (2008) showed that legume N2 fixation was positively correlated with shoot DM and grain yield, which is consistent with the results of this study (Fig. 5C, D). However, high relative ureide-N abundance did not translate into in high grain yield in some legumes, and vice versa (Table 2 and 4). For example, jack bean showed the lowest relative ureide-N and the highest grain yield, while cowpea recorded much higher relative-ureide-N but produced low grain yield. This can be attributed to the instantaneous nature of the ureide method, as it reflects N2 fixation at the time of the sampling, and not over the entire growth period of the plant (Unkovich et al. 2008). However, the positive correlation found between %RU–N and %Ndfa (Fig. 5E) has confirmed the robustness of the 15N abundance technique and the ureide assay in measuring N2 fixation in members of the tribe Phaseoleae (Mohammed et al. 2022).
In conclusion, the results of this study showed that cropping system has an effect on plant growth, symbiotic performance and grain yield. Legumes grown under monocropping recorded higher plant growth, symbiotic performance and grain yield when compared to those grown in mixed cultures. However, shoot %Ndfa was much higher under intercropping than monocropping due to competition by cereal and legume for soil N which generally forces the legume to symbiotically fix more N2. The components of maize yield were similar for the two cropping systems. The 15N natural abundance technique and ureide method were complementary in measuring N2 fixation in members of the tribe Phaseoleae.
Data Availability
Data are contained within the article.
References
Adeleke MA, Haruna IM (2012) Residual nitrogen contributions from grain legumes to the growth and development of succeeding maize crop. ISRN Agronomy 2012:1–5. https://doi.org/10.5402/2012/213729
Alhassan GA, Egbe MO (2014) Bambara groundnut/maize intercropping: Effects of planting densities in Southern guinea savanna of Nigeria. Afr J Agric Res 9:479–486
Beyan SM, Wolde-meskel E, Dakora FD (2018) An assessment of plant growth and N2 fixation in soybean genotypes grown in uninoculated soils collected from different locations in Ethiopia. Symbiosis 75:189–203. https://doi.org/10.1007/s13199-018-0540-9
Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125:1075–1080
Cataldo D, Haroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80
Dahmardeh M, Ghanbari A, Syahsar BA, Ramrodi M (2010) The role of intercropping maize (Zea mays L.) and Cowpea (Vigna unguiculata L.) on yield and soil chemical properties. Afr J Agric Res 5:631–636. https://doi.org/10.5897/AJAR09.607
Dlamini ST, Jaiswal SK, Mohammed M, Dakora FD (2021) Studies of phylogeny, symbiotic functioning and ecological traits of indigenous microsymbionts nodulating Bambara groundnut (Vigna subterranea L. Verdc) in Eswatini. Microb Ecol 1:1–6
Edje OT, Ossom EM (2009) Crop science handbook. Blue Moon Publishers, Manzini, Swaziland
Egbe MO, Alhassan GA, Ijoyah M (2013) Nodulation, nitrogen yield and fixation by bambara groundnut (Vigna subterranea (L.) Verdc.) landraces intercropped with cowpea and maize in Southern Guinea Savanna of Nigeria. Agric Sci 1:15–28
Ghanbari-Bonjar A, Lee HC (2003) Intercropped wheat (Triticum aestivum L.) and bean (Vicia faba L.) as a whole-crop forage: effect of harvest time on forage yield and quality. Grass Forage Sci 58:28–36
Giller KE (2001) Nitrogen fixation in tropical cropping systems. CABI Publishing, Wallingford
Giller KE, Ormesher J, Awah FM (1991) Nitrogen transfer from Phaseolus bean to intercropped maize measured using 15N-enrichment and 15N-isotope dilution methods. Soil Biol Biochem 23:339–346
Hassan MR, Amodu JT, Muhammad IR et al (2014) Forage yield and quality of lablab (Lablab purpureus L. Sweet) intercropped with maize (Zea mays L.) with flooded irrigation system in the semi-arid zone of Nigeria. J Agric Sci 6:196–211. https://doi.org/10.5539/jas.v6n11p196
Hayat R, Ali S, Sohail Ijaz S et al (2008) Estimation of N2-fixation of mung bean and mash bean through xylem ureide technique under rainfed conditions. Pak J Bot 40:723–734
Herridge DF, Giller KE (2016) Measurement of nitrogen fixation. In: Howieson JG, Dilworth MJ (eds) Working with rhizobia. Australian Centre for International Agricultural Research, Canberra, pp 187–220
Herridge DF, Peoples MB (1990) Ureide assay for measuring nitrogen fixation by nodulated soybean calibrated by 15N methods. Plant Physiol 93:495–503
Kermah M, Franke AC, Adjei-Nsiah S et al (2017) Maize-grain legume intercropping for enhanced resource use efficiency and crop productivity in the Guinea savanna of northern Ghana. Field Crops Res 213:38–50. https://doi.org/10.1016/j.fcr.2017.07.008
Kermah M, Franke AC, Adjei-Nsiah S et al (2018) N2-fixation and N contribution by grain legumes under different soil fertility status and cropping systems in the Guinea savanna of northern Ghana. Agric Ecosyst Environ 261:201–210. https://doi.org/10.1016/j.agee.2017.08.028
Kunene TR, Masarirambi MT, Wahome PK, Oseni TO (2019) Influence of kraal manure, chicken manure and inorganic fertilizer on growth, yield and post-harvest quality of pepper (Capsicum annuum L.) in a Sub-tropical environment. Asian J Adv Agric Res 1–11. https://doi.org/10.9734/ajaar/2019/v11i130043
Lengwati DM, Mathews C, Dakora FD (2020) Rotation Benefits From N2-Fixing Grain Legumes to Cereals: From Increases in Seed Yield and Quality to Greater Household Cash-Income by a Following Maize Crop. Front Sustain Food Syst 4:1–16. https://doi.org/10.3389/fsufs.2020.00094
Li L, Yang S, Li X et al (1999) Interspecific complementary and competitive interactions between intercropped maize and faba bean. Plant Soil 212:105–114
Li QZ, Sun JH, Wei XJ et al (2011) Overyielding and interspecific interactions mediated by nitrogen fertilization in strip intercropping of maize with faba bean, wheat and barley. Plant Soil 339:147–161
Mafongoya PL, Bationo A, Kihara J, Waswa BS (2006) Appropriate technologies to replenish soil fertility in southern Africa. Nutr Cycl Agroecosyst 76:137–151. https://doi.org/10.1007/s10705-006-9049-3
Maitra S, Shankar T, Banerjee P (2020) Potential and advantages of maize-legume intercropping system. In: Hossain A (ed) Maize-production and use. Bangladesh Wheat and Maize Research Institute (BWMRI), Dinajpur, pp 103–116
Manasa P (2020) Yield attributes, yield, competitive ability and economics of summer maize-legume intercropping system. Int J Agric Enviro and Biotechnol 13:33–38. https://doi.org/10.30954/0974-1712.1.2020.16
Mohale KC, Belane AK, Dakora FD (2014) Symbiotic N nutrition, C assimilation, and plant water use efficiency in Bambara groundnut (Vigna subterranea L. Verdc) grown in farmers’ fields in South Africa, measured using 15N and 13C natural abundance. Biol Fertil Soils 50:307–319
Mohammed M, Mbah GC, Sowley ENK, Dakora FD (2022) Cowpea genotypic variations in N2 fixation, water use efficiency (δ13C), and grain yield in response to Bradyrhizobium inoculation in the field, measured using xylem n solutes, 15N, and 13C natural abundance. Front Agron 4:1–24. https://doi.org/10.3389/fagro.2022.764070
Mokgehle SN, Dakora FD, Mathews C (2014) Variation in N2 fixation and N contribution by 25 groundnut (Arachis hypogaea L.) varieties grown in different agro-ecologies, measured using 15N natural abundance. Agric Ecosyst Environ 195:161–172. https://doi.org/10.1016/j.agee.2014.05.014
Mthembu BE, Everson TM, Everson CS (2018) Intercropping maize (Zea mays L.) with lablab (Lablab purpureus L.) for sustainable fodder production and quality in smallholder rural farming systems in South Africa. Agroecol Sustain Food Syst 42:362–382. https://doi.org/10.1080/21683565.2017.1393649
Nasar J, Shao Z, Arshad A et al (2020) The effect of maize–alfalfa intercropping on the physiological characteristics, nitrogen uptake and yield of maize. Plant Biol 22:1140–1149. https://doi.org/10.1111/plb.13157
Peoples MB, Faizah AW, Rerkasem B, Herridge DF (1989) Methods for evaluating nitrogen fixation by nodulated legumes in the field. Australian Centre for International Agricultural Research, Canberra
Rusinamhodzi L, Corbeels M, Nyamangara J, Giller KE (2012) Maize-grain legume intercropping is an attractive option for ecological intensification that reduces climatic risk for smallholder farmers in central Mozambique. Field Crops Res 136:12–22. https://doi.org/10.1016/j.fcr.2012.07.014
Shen Q, Chu G (2004) Bi-directional nitrogen transfer in an intercropping system of peanut with rice cultivated in aerobic soil. Biol Fertil Soils 40:81–87. https://doi.org/10.1007/s00374-004-0737-3
Tsubo M, Walker S, Ogindo HO (2005) A simulation model of cereal-legume intercropping systems for semi-arid regions: I. Model Development Field Crops Res 93:10–22. https://doi.org/10.1016/j.fcr.2004.09.002
Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey R, Giller K, Alves B, Chalk P (2008) Measuring plant-associated nitrogen fixation in agricultural systems. Australian Centre for International Agricultural Research, Canberra
Willey RW (1979) Intercropping: its importance and its research needs, Part 2. Agron Relatsh Field Crop Abstracts 32:73–85
Young GBE, Conway CF (1942) On the estimation of allantoin by the Rimini-Schryver reaction. J Biol Chem 142:839–853
Zhang X, Huang G, Zhao Q (2014) Differences in maize physiological characteristics, Nitrogen accumulation, and yield under different cropping patterns and nitrogen levels. Chil J Agric Res 74:326–332. https://doi.org/10.4067/S0718-58392014000300011
Acknowledgements
We are grateful to the National Research Foundation grant number PDG21040959 3370, Tshwane University of Technology, and the South African Research Chair in Agrochemurgy and Plant Symbioses for financial support. The authors also appreciate support from Mr. Manana and all staff of the Malkerns Research Station in Eswatini for helping with field experiments.
Funding
Open access funding provided by Tshwane University of Technology.
Author information
Authors and Affiliations
Contributions
Z.D.N. collected and analysed data, and drafted the manuscript. M.M. took part data collection and analysis and drafted the manuscript with Z.D.N. F.D.D. was the post-doctoral host of Z.D.N., and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ngwenya, Z.D., Mohammed, M. & Dakora, F.D. Monocropping and Intercropping of Maize with Six Food Legumes at Malkerns in Eswatini: Their Effects on Plant Growth, Grain Yield and N2 Fixation, Measured using the 15N Natural Abundance and Ureide Techniques. Symbiosis 92, 257–269 (2024). https://doi.org/10.1007/s13199-024-00971-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-024-00971-x