Abstract
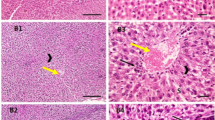
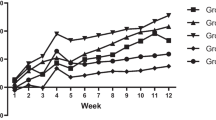
The sources of bioavailable vitamin B12 are limited, and most of them are animal-derived. Chlorella vulgaris, a freshwater microalga, is known for immune system boosting, nutraceutical properties and presence of a natural form of vitamin B12. The present study focused on the in vivo evaluation of the Chlorella biomass as a source of bioavailable vitamin B12 to alleviate the vitamin B12 deficiency status of Wistar rats. Experimental animals were evaluated for the vitamin B12 deficiency-related circulatory marker (serum vitamin B12) and functional markers (plasma homocysteine and urinary methylmalonic acid), haematological and histological changes. The results showed that an increase of 2.4-fold in urinary methylmalonic acid (13.01 ± 0.89 µmoles moles of creatinine−1), 2.6-fold in plasma homocysteine (17.18 ± 3.57 µmole L−1), and 48% decrease in serum vitamin B12 levels (252.69 ± 1.46 pg mL−1) in vitamin B12 deficient group compared to control animals. The Chlorella biomass supplementation in the diet led to the restoration of the functional and circulatory markers, hematological parameters, and vitamin B12 content of kidney and liver to control levels. The Chlorella biomass supplementation increased the erythrocyte precursors and MAST cells in the bone marrow and also normalized the histological features of kidney, liver, and lung tissues. The results suggest that the vitamin B12 from the Chlorella biomass was bioavailable and facilitated the improvement of vitamin B12 status in deficient rats.
Graphic abstract




Similar content being viewed by others
Abbreviations
- AIN:
-
American Institute of Nutrition
- CPCSEA:
-
Committee for the Purpose of Control and Supervision of Experiments on Animals
- GRAS:
-
Generally Regarded As Safe
- IAEC:
-
Institutional Animal Ethics Committee
- IFCC:
-
International Federation of Clinical Chemistry and Laboratory Medicine
- HCT:
-
Hematocrit
- Hcy:
-
Homocysteine
- HGB:
-
Hemoglobin
- KCN:
-
Potassium cyanide
- MCH:
-
Mean corpuscular haemoglobin
- MCHC:
-
Mean corpuscular haemoglobin concentration
- MCV:
-
Mean corpuscular volume
- MMA:
-
Methyl malonic acid
- PC:
-
Platelet count
- PCV:
-
Packed cell volume
- RBC:
-
Red blood cells
- SGOT:
-
Serum glutamic oxaloacetic transaminase
- SGPT:
-
Serum glutamic pyruvic transaminase
- WBC:
-
White blood cells
References
Adaikalakoteswari A, Finer S, Voyias PD et al (2015) Vitamin B12 greater insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin Epigenetics. https://doi.org/10.1186/s13148-015-0046-8
Adaikalakoteswari A, Vatish M, Alam MT et al (2017) Low Vitamin B12 in pregnancy is associated with adipose-derived circulating miRs Targeting PPAR γ and insulin resistance. J ClinEndocrinolMetab 102:4200–4209
Aktas G, Şit M, Tekce H et al (2014) Effects of vitamin B12 treatment on hematological parameters. Acta Med Anatol 2:6–8
AOAC (2000) Official methods of analysis of AOAC International, 17th edn. AOAC International, Gaithersburg
Dryden LP, Hartman AM (1966) Relative concentration of vitamin B12 in the organs of the male rat as affected by its intake of the vitamin. J Nutr 90:382–386
Edelmann M, Aalto S, Chamlagain B et al (2019) Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J Food Compos Anal 82:103226
FSSAI (2016), Food safety and standards (health supplements, nutraceuticals, food for special dietary use, food for special medical purpose, functional food and novel food) regulations, 2016, The Gazette of India, New Delhi. 126
Halle I, Janczyk P, Freyer G, Souffrant WB (2009) Effect of microalgae Chlorella vulgaris on laying hen performance. ArchivaZootechnica 122:5–13
Herrmann W, Obeid R (2008) Causes and early diagnosis of vitamin B12 deficiency. DeutschesÄrzteblattInt 105:680
Janczyk P, Wolf C, Wolfgang BS (2005) Evaluation of nutritional value and safety of the green microalgae Chlorella vulgaris treated with novel processing methods. Arch Zootech 8:132–147
Kamath APS (2017) Methylcobalamin in Vitamin B12 Deficiency: to give or not to give? J PharmacolPharmacother 8:33–34. https://doi.org/10.4103/jpp.JPP
Kim GS, Kim CH, Park JY et al (1996) Effects of vitamin B12 on cell proliferation and cellular alkaline phosphatase activity in human bone marrow stromal osteoprogenitor cells and UMR106 osteoblastic cells. MetabolClinExp 45:1443–1446. https://doi.org/10.1016/S0026-0495(96)90171-7
Kumudha A, Sarada R (2015) Effect of different extraction methods on vitamin B12 from blue green algae, Spirulina platensis. Pharm Anal Acta 06:2–7. https://doi.org/10.4172/2153-2435.1000337
Kumudha A, Selvakumar S, Dilshad P et al (2015) Methylcobalamin: a form of vitamin B12 identified and characterised in Chlorella vulgaris. Food Chem 170:316–320. https://doi.org/10.1016/j.foodchem.2014.08.035
Ling CT, Chow BF (1954) The influence of vitamin B12 on carbohydrate and lipide metabolism. J BiolChem 206:797–805
Madhubalaji CK, Chandra TS, Chauhan VS et al (2020) Chlorella vulgaris cultivation in airlift photobioreactor with transparent draft tube: effect of hydrodynamics, light and carbon dioxide on biochemical profile particularly Omega-6/omega-3 fatty acid ratio. J Food SciTechnol 57:866–876
Madhubalaji CK, Rashmi V, Chauhan VS et al (2019) Improvement of vitamin B12 status with Spirulina supplementation in Wistar rats validated through functional and circulatory markers. J Food Biochem. https://doi.org/10.1111/jfbc.13038
Moll R, Davis B (2017) Iron, vitamin B12 and folate. Medicine 45:198–203. https://doi.org/10.1016/j.mpmed.2017.01.007
Niroula A, Khatri S, Timilsina R et al (2019) Profile of chlorophylls and carotenoids of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) microgreens. J Food SciTechnol 56:2758–2763
Noda K, Ohno N, Tanaka K et al (1996) A water-soluble antitumor glycoprotein from Chlorella vulgaris. Planta Med 62:423–426. https://doi.org/10.1055/s-2006-957931
Paul C, Brady DM (2017) Comparative bioavailability and utilization of particular forms of B12 supplements with potential to mitigate B12-related genetic polymorphisms. Integr Med 16:42–49
Pawlak R, Lester SE, Babatunde T (2014) The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J ClinNutr 68:541–548. https://doi.org/10.1038/ejcn.2014.46
Piyathilake CJ, Johanning GL, Macaluso M et al (2000) Localized folate and vitamin B12 deficiency in squamous cell lung cancer is associated with global DNA hypomethylation. Nutr Cancer 37:99–107
Pugalendhi P, Manoharan S, Panjamurthy K et al (2009) Antigenotoxic effect of genistein against 7,12-dimethylbenz[a]anthracene induced genotoxicity in bone marrow cells of female wistar rats. Pharmacol Rep 61:296–303. https://doi.org/10.1016/S1734-1140(09)70035-0
Reeves PG (1997) Components of the AIN-93 diets as improvements in the AIN-76A Diet. J Nutr 127:838–841
Sandeep Tak M, RajkumarRathore M, Ravi Mangalia M (2019) METformin use reduces serum vitamin B12 levels in dose-dependent and time dependent manner in Asian-Indians with type 2 diabetes. EndocrPract 25:147–147
Stabler SP, Allen RH (2004) Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr. https://doi.org/10.1146/annurev.nutr.24.012003.132440
Tanaka K, Yamada A, Noda K (1997) Oral administration of a unicellular green algae, Chlorella vulgaris, prevents stress-induced ulcer. Planta Med 63:465–466
Tetsunori KM, Takada T et al (1992) Effects of vitamin B12 deficiency on testes tissue in Rats. J NutrSciVitam 38:305–316
VandenBerg H, Brandsen L, Sinkeldam BJ (1991) Vitamin B12 content and bioavailability of Spirulina and nori in rats. J NutrBiochem 2:314–318. https://doi.org/10.1016/0955-2863(91)90073-E
Vidyashankar S, Deviprasad K, Chauhan VS, Sarada R (2013) Selection and evaluation of CO2 tolerant indigenous microalga Scenedesmus dimorphus for unsaturated fatty acid rich lipid production under different culture conditions. BioresourTechnol 144:28–37
Watanabe F (2007) Vitamin B12 sources and bioavailability. ExpBiol Med 232:1266–1274. https://doi.org/10.3181/0703-MR-67
Watanabe F, Takenaka S, Kittaka-Katsura H et al (2002) Characterization and bioavailability of vitamin B12 compounds from edible algae. J NutrSciVitam 48:325–331. https://doi.org/10.3177/jnsv.48.325
Watanabe F, Yabuta Y, Bito T, Teng F (2014) Vitamin B12-containing plant food sources for vegetarians. Nutrients 6:1861–1873
Yetley EA, Pfeiffer CM, Phinney KW et al (2011) Biomarkers of vitamin B12 status in NHANES: a roundtable summary. Am J ClinNutr 94:313S-321S
Acknowledgements
CKMB acknowledges CSIR, Government of India for the award of Senior Research Fellowship (31/5(543)/2017-EMR-I). The authors are thankful to Dr H P Ramesh for his help in the histopathological findings. The authors thank Dr. Shylaja M Dharmesh for her valuable inputs. Authors also thank the Director of CSIR-Central Food Technological Research Institute, Mysore, Karnataka, India. The authors thank the DBT, Govt. of India, New Delhi, India, for providing a research grant (BT/PR10658/PFN/20/806/2013).
Funding
The authors thank the DBT, Govt. of India, New Delhi, India, for providing a research Grant (BT/PR10658/PFN/20/806/2013) to carry out this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Madhubalaji, C.K., Rashmi, V., Chauhan, V.S. et al. Improvement in vitamin B12 status of Wistar rats by supplementing the diet with Chlorella vulgaris biomass. J Food Sci Technol 58, 4270–4281 (2021). https://doi.org/10.1007/s13197-020-04901-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04901-9