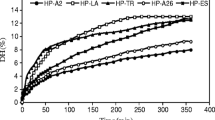
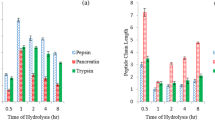
Abstract
The antioxidative capacity of six different tissue hydrolysates (porcine colon, heart and neck and bovine lung, kidney and pancreas) were tested by three different assays monitoring iron chelation, ABTS radical scavenging and inhibition of lipid oxidation in emulsions, respectively. The hydrolysates were also investigated with respect to amino acid composition and peptide size distribution. The hydrolysates contained peptides ranging from 20 kDa to below 100 Da with a predominance of peptides with low molecular weight (53.8 to 89.0 % below 3 kDa). All hydrolysates exhibited antioxidant activity as assessed with all three methods; inhibition of lipid oxidation ranging from 72 to 88 % (at a final protein concentration of 7 mg/mL), iron chelation capacity from 23 to 63 % (at 1.1 mg/mL), and ABTS radical scavenging from 38 to 50 % (at 10 μg /mL). The antioxidant activity did not correlate with the proportion of low molecular weight peptides in the hydrolysed tissues, but with the content of specific amino acid residues. The ABTS radical scavenging capacity of the tissues was found to correlate with the content of Trp, Tyr, Met and Arg, whereas the ability to inhibit the oxidation of lineoleic acid correlated with the content of Glu and His. The chosen animal by-products thus represent a natural source of antioxidants with potential for food application.


Similar content being viewed by others
References
Ajibola CF, Fashakin JB, Fagbemi TN, Aluko RE (2011) Effect of peptide size on antioxidant properties of african yam bean seed (sphenostylis stenocarpa) protein hydrolysate fractions. Int J Mol Sci 12:6685–6702
Anderson BA (1988) Composition and nutritional value of edible meat by-products. In: Pearson AM, Dutson TR (eds) Edible meat by-products advances in meat research. Elsevier Science Publishers Ltd, Amsterdam, pp 15–45
Balakrishnan B, Prasad B, Rai AK, Velappan SP, Subbanna MN, Narayan B (2011) In vitro antioxidant and antibacterial properties of hydrolysed proteins of delimed tannery fleshings: comparison of acid hydrolysis and fermentation methods. Biodegradation 22:287–295
Chaiyasit W, Fau ER, Fau MD, Decker EA (2007) Role of physical structures in bulk oils on lipid oxidation. Crit Rev Food Sci Nutr 47:299–317
Chan WKM, Decker EA, Lee JB, Butterfield DA (1994) EPR spin-trapping studies of the hydroxyl radical scavenging activity of carnosine and related dipeptides. J Agric Food Chem 42:1407–1410
Damgaard TD, Otte J, Meinert L, Jensen K, Lametsch R (2014) Antioxidant capacity of hydrolyzed porcine tissues. Food Sci Nutr 2:282–288
De Gobba C, Tompa G, Otte J (2014) Bioactive peptides from caseins released by cold active proteolytic enzymes from Arsukibacterium ikkense. Food Chem 165:205–215
Di Bernardini R, Rai DK, Bolton D, Kerry J, O’Neill E, Mullen AM, Hayes M (2011) Isolation, purification and characterization of antioxidant peptidic fractions from a bovine liver sarcoplasmic protein thermolysin hydrolysate. Peptides 32:388–400
Donnelly JL, Decker EA, McClements DJ (1998) Iron-catalyzed oxidation of menhaden oil as affected by emulsifiers. J Food Sci 63:997–1000
Eastoe JE (1955) The amino acid composition of mammalian collagen and gelatin. Biochem J 61:589–600
Fan J, He J, Zhuang Y, Sun L (2012) Purification and identification of antioxidant peptides from enzymatic hydrolysates of tilapia (oreochromis niloticus) frame protein. Molecules 17:12836–12850
Farvin KHS, Andersen LL, Nielsen HH, Jacobsen C, Jakobsen G, Johansson I, Jessen F (2014) Antioxidant activity of cod (gadus morhua) protein hydrolysates: in vitro assays and evaluation in 5 % fish oil-in-water emulsion. Food Chem 149:326–334
Gault NF, Lawrie RA (1980) Efficiency of protein extraction and recovery from meat industry by-products. Meat Sci 4:167–190
Guo H, Kouzuma Y, Yonekura M (2009) Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem 113:238–245
Hernandez-Ledesma B, Davalos A, Bartolome B, Amigo L (2005) Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin identification of active peptides by HPLC-MS/MS. J Agric Food Chem 53:588–593
Irshad I, Kanekanian A, Peters A, Masud T (2013) Antioxidant activity of bioactive peptides derived from bovine casein hydrolysate fractions. J Food Sci Technol:1–9
Joseph TR, Reynolds PR, Bolton D, Fitzgerald GF, Stanton C (2011) Bioactive peptides from muscle sources: meat and fish. Nutrients 3:765–791
Kim YJ, Lee KW, Lee HJ (2004) Total antioxidant capacity of arginine-conjugated linoleic acid (CLA) complex. J Agric Food Chem 52:439–444
Kristiansen KR, Otte J, Ipsen R, Qvist KB (1998) Large-scale preparation of β-lactoglobulin A and B by ultrafiltration and ion-exchange chromatography. Int Dairy J 8:113–118
Lass A, Suessenbacher A, Wölkart G, Mayer B, Brunner F (2002) Functional and analytical evidence for scavenging of oxygen radicals by l-arginine. Mol Pharmacol 61:1081–1088
Liu Q, Kong B, Xiong YL, Xia X (2010) Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem 118:403–410
Najafian L, Babji AS (2014) Production of bioactive peptides using enzymatic hydrolysis and identification antioxidative peptides from patin (pangasius sutchi) sarcoplasmic protein hydolysate. J Funct Foods 9:280–289
Nasri R, Younes I, Jridi M, Trigui M, Bougatef A, Nedjar-Arroume N, Dhulster P, Nasri M, Karra-Châabouni M (2013) ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: effect on meat lipid oxidation. Food Res Int 54:552–561
Pihlanto A (2006) Antioxidative peptides derived from milk proteins. Int Dairy J 16:1306–1314
Sachindra NM, Bhaskar N (2008) In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour Technol 99:9013–9016
Saiga E, Tanabe S, Nishimura T (2003) Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem 51:3661–3667
Saito K, Jin D, Ogawa T, Muramoto K, Hatakeyama E, Yasuhara T, Nokihara K (2003) Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J Agric Food Chem 51:3668–3674
Samaranayaka AGP, Li-Chan ECY (2011) Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. J Funct Foods 3:229–254
Sarmadi BH, Ismail A (2010) Antioxidative peptides from food proteins: a review. Peptides 31:1949–1956
Schweigert BS, Bennet BA, Guthneck BT (1954) Amino acid composition of organ meats. J Food Sci 19:219–223
Sharp JS, Becker JM, Hettich RL (2004) Analysis of protein solvent accessible surfaces by photochemical oxidation and mass spectrometry. Anal Chem 76:672–683
Storcksdieck S, Bonsmann G, Hurrell RF (2007) Iron-binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources. J Food Sci 72:S019–S029
Torres-Fuentes C, Alaiz M, Vioque J (2012) Iron-chelating activity of chickpea protein hydrolysate peptides. Food Chem 134:1585–1588
Udenigwe CC, Aluko RE (2011) Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci 77:R11–R24
Xiong YL (2010) Antioxidant peptides. In: Mine Y, Li-Chan E, Jiang B (eds) Bioactive proteins and peptides as functional foods and nutraceuticals. Blackwell Publishing Ltd, Amsterdam, pp 29–42
Acknowledgments
Thanks to Erik T. Hansen and Kim Dam Nielsen from Dat-Schaub for producing the hydrolysates and for providing support and help. Thanks to Lene Meinert from the Danish Meat Research Institute for data sharing, support and help, and thanks to Daniel Tsegay Berhe from Food Science, University of Copenhagen, for help with the PCA analysis. This work was financed by inSPIRe and the Pig Levy Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Damgaard, T., Lametsch, R. & Otte, J. Antioxidant capacity of hydrolyzed animal by-products and relation to amino acid composition and peptide size distribution. J Food Sci Technol 52, 6511–6519 (2015). https://doi.org/10.1007/s13197-015-1745-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1745-z