Abstract
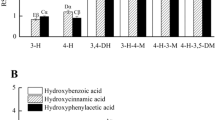
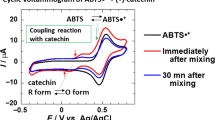
The free radical scavenging activity and reducing power of 16 phenolic compounds including four hydroxycinnamic acid derivatives namely ferulic acid, caffeic acid, sinapic acid and p-coumaric acid, benzoic acid and its derivatives namely protocatechuic acid, gallic acid and vanillic acid, benzene derivatives namely vanillin, vanillyl alcohol, veratryl alcohol, veratraldehyde, pyrogallol, guaiacol and two synthetic antioxidants, butylated hydroxy anisole (BHA) and propyl gallate were evaluated using 1,1-Diphenyl-2-picrylhydrazyl radical (DPPH•), 2,2′-Azinobis-3- ethylbenzothiazoline-6-sulfonic acid radical (ABTS+•), Hydroxyl radical (•OH) and Superoxide radical (O2 •-) scavenging assays and reduction potential assay. By virtue of their hydrogen donating ability, phenolic compounds with multiple hydroxyl groups such as protocatechuic acid, pyrogallol, caffeic acid, gallic acid and propyl gallate exhibited higher free radical scavenging activity especially against DPPH• and O2 •-. The hydroxylated cinnamates such as ferulic acid and caffeic acid were in general better scavengers than their benzoic acid counter parts such as vanillic acid and protocatechuic acid. All the phenolic compounds tested exhibited more than 85 % scavenging due to the high reactivity of the hydroxyl radical. Phenolic compounds with multiple hydroxyl groups also exhibited high redox potential. Exploring the radical scavenging and reducing properties of antioxidants especially those which are found naturally in plant sources are of great interest due to their protective roles in biological systems.






Similar content being viewed by others
Abbreviations
- Prot:
-
Protocatechuic acid
- Guai:
-
Guaiacol
- Caff:
-
Caffeic acid
- Feru:
-
Ferulic acid
- Coum:
-
p-Coumaric acid
- Gall:
-
Gallic acid
- Vaci:
-
Vanillic acid
- Sina:
-
Sinapinic acid
- Prop:
-
Propyl gallate
- Veald:
-
Veratraldehyde
- BHA:
-
Butylated hydroxy anisole
- Veal:
-
Veratryl alcohol
- Valc:
-
Vanillyl Alcohol
- Pyro:
-
Pyrogallol
- Benz:
-
Benzoic acid
- Vani:
-
Vanillin
References
Bilto YY, Suboh S, Aburjai T, Abdalla S (2012) Structure activity relationships regarding the antioxidant effects of the flavonoids on human erythrocytes. Natural Sci 4:740–747
Bountagkidou OG, Ordoudi SA, Tsimidou MZ (2010) Structure-antioxidant activity relationship study of natural hydroxybenzaldehydes using in vitro assays. Food Res Int 43:2014–2019
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Cai YZ, Mei S, Jie X, Luo Q, Corke H (2006) Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci 78:2872–2888
Crozier A, Jaganath IB, Clifford MN (2009) Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep 26:1001–1043
Cuvelier ME, Richard H, Berset C (1992) Comparison of the antioxidant activity of some acid phenols: structure-activity relationship. Biosci Biotechnol Biochem 56:324–325
De Ancos B, Gonzàlez EM, Cano MP (2000) Ellagic acid, vitamin C and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J Agric Food Chem 48:4565–4570
Dziedzic SZ, Hudson BJF (1983) Polyhydroxychalcones and flavanones as antioxidants for edible foods. Food Chem 12:205–212
Grootveld M, Halliwell B (1986) Aromatic hydroxylation as a potential measure of hydroxyl radical formation in vivo. Biochem J 237:499–504
Gul MZ, Bhakshu LM, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA (2011) Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using invitro assays. BMC Complement Altern Med 11:64–76
Halliwell B, Gutteridge JMC, Aruoma OI (1987) The deoxyribose method: a simple test tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165:215–219
Haseloff RF, Blasig IE, Meffert H, Ebert B (1990) Hydroxyl radical scavenging and antipsoriatic activity of benzoic acid derivatives. Free Rad Biol Med 9:111–115
Heo BG, Park YJ, Park YS, Bae JH, Cho JY, Park K, Jastrzebski Z, Gorinstein S (2014) Anticancer and antioxidant effects of extracts from different parts of indigo plant. Ind Crop Prod 56:9–16
Hermann K (1989) Occurrence and content of hydroxycinnamic acid and hydroxybenzoic acid compounds in foods. Crit Rev Food Sci Nutr 28:315–347
Hsieh CL, Chang CH, Chiang SY, Li TC, Tang NY, Pon CZ, Hsieh CT, Lin JG (2000) Anticonvulsive and free radical scavenging activities of vanillyl alcohol in ferric chloride-induced epileptic seizures in Sprague–Dawley rats. Life Sci 67:1185–1195
Jing P, Zhao SJ, Jian WJ, Qian BJ, Dong Y, Pang J (2012) Quantitative studies on structure-DPPH scavenging activity relationships of food phenolic acids. Molecules 17:12910–12924
Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H (2002) Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem 50:2161–2168
Kumar SS, Priyadarsini KI, Sainis KB (2002) Free radical scavenging activity of vanillin and o-vanillin using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical. Redox Rep 7:35–40
Kumbulainen JT, Salonen JT (1999) Natural antioxidants and anticarcinogens in nutrition: Health and diseases. Royal Society of Chemistry, Cambridge, p 12
Leonardis AD, Macciola V (2003) Effectiveness of caffeic acid as an antioxidant for cod liver oil. Inter J Food Sci Technol 38:475–480
Lin S, Lai TC, Chen L, Kwok HF, Lau CB, Cheung PCK (2014) Antioxidant and antiangiogenic properties of phenolic extract from Pleurotus tuber-regium. J Agric Food Chem 62:9488–9498
Lipinski B (2011) Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev 809696:1–9
Liu J, Mori A (1993) Antioxidant and pro-oxidant activities of p-hydroxybenzyl alcohol and vanillin: effects on free radicals, brain peroxidation and degradation of benzoate, deoxyribose, amino acids and DNA. Neuropharmacol 32:659–669
Lucini L, Pellizoni M, Pellegrino R, Molinari GP, Colla G (2015) Phytochemical constituents and in vitro radical scavenging activity of different Aloe species. Food Chem 170:501–507
Marinova EM, Yanishlieva NV (1992) Effect of temperature on the antioxidative action of inhibitors in lipid autoxidation. J Sci Food Agric 60:313–318
Mathew S, Abraham TE (2006) Invitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol 44:198–206
Matthaus B (2002) Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem 50:3444–3452
Moure A, Cruz JM, Franco D (2001) Natural antioxidants from residual sources. Food Chem 72:145–171
Natella F, Nardini M, di Felice M, Scaccini C (1999) Benzoic and cinnamic acid derivatives as antioxidants: Structure-activity relation. J Agric Food Chem 47:1453–1459
Nenadis N, Wang LF, Tsimidou M, Zhang HY (2004) Estimation of scavenging activity of phenolic compounds using the ABTS assay. J Agric Food Chem 52:4669–4674
Nicodemus KK, Jacobs DR, Folsom AR (2001) Whole and refined grain intake and risk of incident postmenopausal breast cancer. Cancer Cause Control 12:917–925
Pacifico S, Gallicchio M, Lorenz P, Duckstein SM, Potenza N, Galasso S, Marciano S, Fiorentino A, Stintzing FC, Monaco P (2014) Neuroprotective potential of Laurus nobilis antioxidant polyphenol enriched leaf extracts. Chem Res Toxicol 27:611–626
Pino E, Campos AM, Lopez-Alarcon C, Aspee A, Lissi E (2006) Free radical scavenging capacity of hydroxycinnamic acids and related compounds. J Phys Org Chem 19:759–764
Pokorny J (1987) Major factors affecting the autooxidation of lipids. In: Chan HWS (ed) Autooxidation of unsaturated lipids. Academic, London, pp 141–206
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radical Biol Med 26:1231–1237
Rice Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med 20:933–956
Robbins RJ (2003) Phenolic acids in foods: An overview of analytical methodology. J Agric Food Chem 51:2866–2887
Shahidi F, Wanasundara PKJ (1992) Phenolic antioxidants. Crit Rev Food Sci Nutr 32:67–103
Siquet C, Paiva-Martins F, Lima JL, Reis S, Borges F (2006) Antioxidant profile of dihydroxy- and trihydroxyphenolic acids–a structure activity relationship study. Free Radical Res 40:433–442
Sivakumar PM, Prabhakar PA, Doble M (2011) Synthesis, antioxidant evaluation and quantitative structure-activity relationship studies of chalcones. Med Chem Res 20:482–492
Teixeira J, Gaspar A, Garrido EM, Borges F (2013) Hydroxycinnamic acid antioxidants: An electrochemical overview. Biomed Res Int 2013, Article ID 251754, 11 pages
Torres de Pinedo A, Penalver P, Morales JC (2007) Synthesis and evaluation of new phenolic-based antioxidants: structure-activity relationship. Food Chem 103:55–61
Vafiadis AP, Bakalbassis EG (2003) A computational study of the structure–activity relationships of some p-hydroxybenzoic acid antioxidants. J Am Oil Chem Soc 80:1217–1223
Velkov ZA, Kolev MK, Tadjer AV (2007) Modeling and statistical analysis of DPPH scavenging activity of phenolics. Collec Czech Chem Commun 72:1461–1471
von Gadow A, Joubert E, Hansmann CF (1997) Comparison of the antioxidant activity of aspalathin with that of other plant phenols of Rooibos tea (Aspalathus linearis), alpha-Tocopherol, BHT, and BHA. J Agric Food Chem 45:632–638
Wardman P (1989) Reduction potentials of one-electron couples involving free radicals in aqueous solutions. J Phys Chem Ref Data 18:1637–1755
Yamagami C, Akamatsu M, Motohashi N, Hamada S, Tanahashi T (2005) Quantitative structure-activity relationship studies for antioxidant hydroxybenzalacetones by quantum chemical-and 3-D-QSAR(CoMFA) analyses. Bioorg Med Chem Lett 15:2845–2850
Yanishlieva N, Marinova EM (1995) Effects of antioxidants on the stability of triacylglycerols and methyl esters of fatty acids of sunflower oil. Food Chem 54:377–382
Zhang J, Stanley RA, Adaim A, Melton LD, Skinner MA (2006) Free radical scavenging and cytoprotective activities of phenolic antioxidants. Mol Nutr Food Res 50:996–1005
Acknowledgments
The authors are thankful to the Director, NIIST (CSIR), India for providing the facilities for carrying out this research work. The first author acknowledges CSIR, India for the Research Fellowship and Universiti Teknologi Malaysia (UTM) for the Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathew, S., Abraham, T.E. & Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Technol 52, 5790–5798 (2015). https://doi.org/10.1007/s13197-014-1704-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1704-0