Abstract
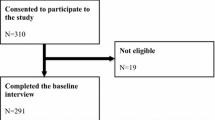
The purpose of this study is to validate the Brazilian version of Functional Assessment of Cancer Therapy-Prostate FACT-P (version 4) in nonmetastatic prostate cancer (PC) patients. Patients with histopathological diagnosis of PC were submitted to health-related quality of life (HRQOL) questionnaires – SF-36 (Medical Outcomes Study 36 – Item Short-Form Health Survey) and FACT-P (version 4). After 7 to 15 days, FACT-P (version 4) was reapplied in the sample’s percentage that participated the first evaluation. Cronbach alpha coefficient was used to determine internal consistency and intraclass correlation coefficient (ICC) certified stability. Correlations between FACT-P (version 4) and SF-36 tested convergent validity. Regarding known groups validity, the hypothesis tested was that FACT-P (version 4) is capable of discriminating HRQOL in patients with different PC risk classifications. A total of 112 patients with nonmetastatic PC were evaluated. Cronbach alpha coefficients (0.64–0.88) and ICC (0.75–0.93) obtained satisfactory results of reliability. Verified correlations (r 0.3–0.72) between FACT-P (version 4) and SF-36 suggest convergent validity. In the studied sample, FACT-P (version 4) was unable to discriminate HRQOL in nonmetastatic patients. The Brazilian version of FACT-P questionnaire (version 4) showed evidences of reliability and validity on evaluating HRQOL in Brazilian men with nonmetastatic PC.
Similar content being viewed by others
References
Brasil (2015) Estimativa 2016: Incidência de câncer no Brasil. Instituto Nacional do Câncer (INCA)/Ministério da Saúde - Brasil, Rio de Janeiro, p 122
Mottet N, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 71:618–629
Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smit MR (2008) Management of complications of prostate cancer treatment. CA A Cancer J Clin 58(4):196–213
Stavrinides V, Parker CC, Moore CM (2017) When no treatment is the best treatment: active surveillance strategies for low risk prostate cancers. Cancer Treat Rev 58:14–21
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S (2015) Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 33(3):272–277
American Cancer Society (2015) Cancer facts & figures. American Cancer Society, Atlanta
Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E (2016) Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 375:1425–1437
Lardas M, Liew M, van den Bergh RC et al (2017) Quality of life outcomes after primary treatment for clinically localised prostate cancer: a systematic review. Eur Urol 72(6):869–885
Drummond FJ, Kinnear H, O´Leary E, Donnelly, Gavin A, Sharp L (2015) Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv 9:361–372
Schmidt S, Garin O, Pardo Y, Valderas JM, Alonso J, Rebollo P et al (2014) Assessing quality of life in patients with prostate cancer: a systematic and standardized comparison of available instruments. Qual Life Res 23:2169–2181
Hamoen EHJ, Rooij M, Witjes JA, Barentsz JO, Rovers MM (2015) Measuring health-related quality of life in men with prostate cancer: a systematic review of the most used questionnaires and their validity. Urol Oncol 33(2):69.e19–28
Chi KN, Protheroe A, Rodríguez-Antolín A, Facchini G, Suttman H, Matsubara N et al (2018) Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol 19(2):194–206
Bonomi AE, Celia DF, Hahn EA, Bjordai K, Sperner-Unterweger B, Gangeri L et al (1996) Multilingual translation of the Functional Assessment of Cancer Therapy (FACT) quality of life measurement system. Qual Life Res 5:309–320
Webster K, Cella D, Yost K (2003) The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 1:79
Cella DF, Tulsky DS, Gray G et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11(3):570–579
Esper PEG, Mo FEI, Chodak G, Sinner M, Cella D, Pienta KJ (1997) Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy - prostate instrument. Urology 50(6):920–928
Stenner F, Rothschild SI, Betticher D et al (2017) Quality of life in second-line treatment of metastatic castration-resistant prostate cancer using cabazitaxel or other therapies after previous docetaxel chemotherapy: Swiss Observational Treatment Registry. Clin Genitourin Cancer 16(1):e151-159
Fizazi K, Ulys A, Sengeløv L et al (2017) A randomized, double-blind, placebo-controlled phase II study of maintenance therapy with tasquinimod in patients with metastatic castration-resistant prostate cancer responsive to or stabilized during first-line docetaxel chemotherapy. Ann Oncol 28(11):2741–2746
Aning JJ, MacKenzie KR, Fabricius M et al (2018) Detailed analysis of patient-reported lower urinary tract symptoms and effect on quality of life after robotic radical prostatectomy. Urol Oncol 36(8):364.e15-364.e22
Terwee CB, Bot SDM, Boer MR, Windt AWM, Knol DL, Dekker J et al (2007) Quality criteria were proposed for measurements properties of health status questionnaires. J Clin Epidemiol 60(1):34–42
DeVon HA, Block ME, Moyle-Wright P, Ernst DM, Hayden SJ et al (2007) A psychometric toolbox for testing validity and reliability. J Nurs Scholarsh 39(2):155–164
Polit D, Beck C. Fundamentos de pesquisa em enfermagem: avaliação de evidências para a prática da enfermagem. 2011. 7ª ed. Porto Alegre: Artmed
Hair JF, Black WC, Babin BB, Anderson RE, Tatham RL (2009) Análise multivariada de dados, 6th edn. Bookman, Porto Alegre
Ciconelli RM, Ferraz MB, Santos W et al (1999) Tradução para a língua portuguesa e validação do questionário genérico de avaliação de qualidade de vida SF-36 (Brasil SF-36). Rev Bras Reumatol 39(3):143–150
Bergman J, Laviana A (2014) Quality-of-life assessment tools for men with prostate cancer. Nat Rev Urol 11:352–359
Morris C, Gibbons E, Fitzpatrick R. 2009. A structured review of patient-reported outcome measures (PROMs) for men with prostate cancer. Patient-reported Outcome Measurement Group, Department of Public Health, University of Oxford
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading Commitee (2014) The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 40(2):244–252
Martins GA (2006) Sobre confiabilidade e validade. Revista Brasileira de Gestão de Negócios 8(20):1–12
Portney LG, Watkins MP (2009) Foundations of clinical research: applications to practice, 3rd edn. Pearson/Prentice Hall, Upper Saddle River
Ware JE, Kosinski M, Keller SD (1994) The SF-36 Physical and Mental Health Summary Scales: a user’s manual. The Health Institute, Boston
Pagano M, Gauvreau K (2004) Princípios de Bioestatística. Thomson, São Paulo
Chi KN, Protheroe A, Rodríguez-Antolín A et al (2018) Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol 19(2):194–206
Batista-Miranda JE, Sevilla-Cecilia C, Torrubia R et al (2003) Quality of life in prostate cancer patients and controls: psychometric validation of the FACTP-4 in Spanish, and relation to urinary symptoms. Arch EspUrol 56(4):447–454
Sharpley CF, Bitsika V, Christie DRH (2018) “The worst thing was…”: prostate cancer patients’ evaluations of their diagnosis and treatment experiences. Am J Mens Health 12(5):1503–1509
Incrocci L, Madalinska JB, Essink-Bot M-L, Van Putten WLJ, Koper PCM, Schroeder FH (2001) Sexual functioning in patients with localized prostate cancer awaiting treatment. J Sex Marital Ther 27:353–363
Brown M (2010) Prostate cancer: how assessment of QoL can improve delivery of care. Br J Nurs 19(17):1080–1084
Fregnani CMS, Fregnani JHTG, Latorre MRDO, Almeida AM (2013) Evaluation of the psychometric properties of the Functional Assessment of Cancer Therapy-Cervix questionnaire in Brazil. PlosOne 8(10):1–9
Ishikawa NM, Thuler LC, Giglio AG, Baldotto CS, de Andrade CJ, Derchain SF (2010) Validation of the Portuguese version of Functional Assessment of Cancer Therapy-Fatigue (FACT-F) in Brazilian cancer patients. Support Care Cancer 18(4):481–490
Hong JH, Jeon SS, Lee HM, Choi Y, Kim S, Choi HY (2006) The Functional Assessment of Cancer Therapy-Prostate (FACT-P) scales in men with prostate cancer: reliability and validity of the Korean Version. J Korean Med 21(2):295–299
Wong CKH, Choi EPH, Tsu JHL et al (2015) Psychometric properties of Functional Assessment of Cancer Therapy-Prostate (FACT-P) in Chinese patients with prostate cancer. Qual Life Res 24:2397–2402
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Usage rights of the Portuguese version of FACT-P questionnaire were granted by FACIT organization (Functional Assessment of Chronic Illness Therapy).
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (the State University of Campinas’ Ethics in Research Committee, License number 1.377.772) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Held, P.A., Matheus, W.E., Naccarato, A.M.E.P. et al. Validation of the Brazilian Version of Functional Assessment of Cancer Therapy-Prostate—FACT-P (Version 4) in Prostate Cancer Patients. J Canc Educ 37, 1760–1767 (2022). https://doi.org/10.1007/s13187-021-02024-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13187-021-02024-z