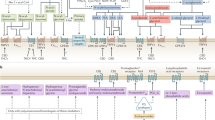
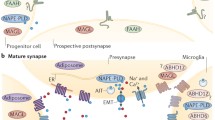
Abstract
Cannabis-inspired medical products are garnering increasing attention from the scientific community, general public, and health policy makers. A plethora of scientific literature demonstrates intricate engagement of the endocannabinoid system with human immunology, psychology, developmental processes, neuronal plasticity, signal transduction, and metabolic regulation. Despite the therapeutic potential, the adverse psychoactive effects and historical stigma, cannabinoids have limited widespread clinical application. Therefore, it is plausible to weigh carefully the beneficial effects of cannabinoids against the potential adverse impacts for every individual. This is where the concept of “personalized medicine” as a promising approach for disease prediction and prevention may take into the account. The goal of this review is to provide an outline of the endocannabinoid system, including endocannabinoid metabolizing pathways, and will progress to a more in-depth discussion of the therapeutic interventions by endocannabinoids in various neurological disorders.

Similar content being viewed by others
References
Badowski ME. A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics. Cancer Chemother Pharmacol. 2017;80(3):441–9. https://doi.org/10.1007/s00280-017-3387-5.
Rock EM, Parker LA. Cannabinoids as potential treatment for chemotherapy-induced nausea and vomiting. Front Pharmacol. 2016;7:221. https://doi.org/10.3389/fphar.2016.00221.
Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–80. https://doi.org/10.1016/S1474-4422(18)30499-X.
Feigin VL, Vos T. Global burden of neurological disorders: from global burden of disease estimates to actions. Neuroepidemiology. 2019;52(1–2):1–2. https://doi.org/10.1159/000495197.
WHO. Global burden of neurological disorders: estimates and projections. In: Neurological disorders: public health challenges. Geneva, Switzerland: WHO Press. https://www.who.int/mental_health/neurology/chapter_2_neuro_disorders_public_h_challenges.pdf?ua=1. Accessed Jan 22 2020.
Abey NO. Cannabis sativa (marijuana) alters blood chemistry and the cytoarchitecture of some organs in Sprague Dawley rat models. Food Chem Toxicol. 2018;116(Pt B):292–7. https://doi.org/10.1016/j.fct.2018.04.023.
Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2(6):241–54. https://doi.org/10.1177/2045125312457586.
Goyal H, Awad HH, Ghali JK. Role of cannabis in cardiovascular disorders. J Thoracic Dis. 2017;9(7):2079–92. https://doi.org/10.21037/jtd.2017.06.104.
Goyal H, Singla U, Gupta U, May E. Role of cannabis in digestive disorders. Eur J Gastroenterol Hepatol. 2017;29(2):135–43. https://doi.org/10.1097/meg.0000000000000779.
Zimmermann K, Walz C, Derckx RT, Kendrick KM, Weber B, Dore B, et al. Emotion regulation deficits in regular marijuana users. Hum Brain Mapp. 2017;38(8):4270–9. https://doi.org/10.1002/hbm.23671.
Yu SJ, Reiner D, Shen H, Wu KJ, Liu QR, Wang Y. Time-dependent protection of CB2 receptor agonist in stroke. PLoS One. 2015;10(7):e0132487. https://doi.org/10.1371/journal.pone.0132487.
Zhang J, Teng Z, Song Y, Hu M, Chen C. Inhibition of monoacylglycerol lipase prevents chronic traumatic encephalopathy-like neuropathology in a mouse model of repetitive mild closed head injury. J Cereb Blood Flow Metab. 2015;35(3):443–53. https://doi.org/10.1038/jcbfm.2014.216.
Zhang M, Martin BR, Adler MW, Razdan RK, Ganea D, Tuma RF. Modulation of the balance between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152(3):753–60. https://doi.org/10.1016/j.neuroscience.2008.01.022.
Ilyasov AA, Milligan CE, Pharr EP, Howlett AC. The endocannabinoid system and oligodendrocytes in health and disease. Front Neurosci. 2018;12(733). https://doi.org/10.3389/fnins.2018.00733.
Braun M, Khan ZT, Khan MB, Kumar M, Ward A, Achyut BR, et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav Immun. 2017. https://doi.org/10.1016/j.bbi.2017.10.021.
Zhou J, Noori H, Burkovskiy I, Lafreniere JD, Kelly MEM, Lehmann C. Modulation of the endocannabinoid system following central nervous system injury. Int J Mol Sci. 2019;20(2). https://doi.org/10.3390/ijms20020388.
Cecilia JH. Role of cannabinoids and endocannabinoids in cerebral ischemia. Curr Pharm Des. 2008;14(23):2347–61. https://doi.org/10.2174/138161208785740054.
Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43(1):211–9. https://doi.org/10.1161/strokeaha.111.631044.
Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros MP, Garcia-Segura LM. Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One. 2015;10(6):e0128782. https://doi.org/10.1371/journal.pone.0128782.
Svizenska IH, Brazda V, Klusakova I, Dubovy P. Bilateral changes of cannabinoid receptor type 2 protein and mRNA in the dorsal root ganglia of a rat neuropathic pain model. J Histochem Cytochem. 2013;61(7):529–47. https://doi.org/10.1369/0022155413491269.
Concannon RM, Okine BN, Finn DP, Dowd E. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp Neurol. 2016;283(Pt A):204–12. https://doi.org/10.1016/j.expneurol.2016.06.014.
Concannon RM, Okine BN, Finn DP, Dowd E. Differential upregulation of the cannabinoid CB(2) receptor in neurotoxic and inflammation-driven rat models of Parkinson’s disease. Exp Neurol. 2015;269:133–41. https://doi.org/10.1016/j.expneurol.2015.04.007.
Aso E, Ferrer I. CB2 cannabinoid receptor as potential target against Alzheimer’s disease. Front Neurosci. 2016;10:243. https://doi.org/10.3389/fnins.2016.00243.
Garcia-Gutierrez MS, Perez-Ortiz JM, Gutierrez-Adan A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol. 2010;160(7):1773–84. https://doi.org/10.1111/j.1476-5381.2010.00819.x.
Garcia-Gutierrez MS, Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol. 2011;25(1):111–20. https://doi.org/10.1177/0269881110379507.
Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology. 2011;36(7):1489–504. https://doi.org/10.1038/npp.2011.34.
Agudelo M, Yndart A, Morrison M, Figueroa G, Munoz K, Samikkannu T, et al. Differential expression and functional role of cannabinoid genes in alcohol users. Drug Alcohol Depend. 2013;133(2):789–93. https://doi.org/10.1016/j.drugalcdep.2013.08.023.
Ortega-Alvaro A, Ternianov A, Aracil-Fernandez A, Navarrete F, Garcia-Gutierrez MS, Manzanares J. Role of cannabinoid CB2 receptor in the reinforcing actions of ethanol. Addict Biol. 2015;20(1):43–55. https://doi.org/10.1111/adb.12076.
Navarrete F, Rodriguez-Arias M, Martin-Garcia E, Navarro D, Garcia-Gutierrez MS, Aguilar MA, et al. Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology. 2013;38(12):2515–24. https://doi.org/10.1038/npp.2013.157.
Buch SJ. Cannabinoid receptor 2 activation: a means to prevent monocyte-endothelium engagement. Am J Pathol. 2013;183(5):1375–7. https://doi.org/10.1016/j.ajpath.2013.08.003.
Ashton CH, Moore PB. Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr Scand. 2011;124(4):250–61. https://doi.org/10.1111/j.1600-0447.2011.01687.x.
Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79(7):516–25. https://doi.org/10.1016/j.biopsych.2015.07.028.
Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–22. https://doi.org/10.1146/annurev.pharmtox.46.120604.141254.
Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. https://doi.org/10.1124/pr.58.3.2.
Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103(36):13345–50. https://doi.org/10.1073/pnas.0601832103.
Simon GM, Cravatt BF. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol BioSyst. 2010;6(8):1411–8. https://doi.org/10.1039/C000237B.
Tsuboi K, Ikematsu N, Uyama T, Deutsch DG, Tokumura A, Ueda N. Biosynthetic pathways of bioactive N-acylethanolamines in brain. CNS Neurol Disord Drug Targets. 2013;12(1):7–16.
Shonesy BC, Winder DG, Patel S, Colbran RJ. The initiation of synaptic 2-AG mobilization requires both an increased supply of diacylglycerol precursor and increased postsynaptic calcium. Neuropharmacology. 2015;91:57–62. https://doi.org/10.1016/j.neuropharm.2014.11.026.
Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72(3):612–21. https://doi.org/10.1124/mol.107.037796.
Murataeva N, Straiker A, Mackie K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol. 2014;171(6):1379–91. https://doi.org/10.1111/bph.12411.
Luk T, Jin W, Zvonok A, Lu D, Lin XZ, Chavkin C, et al. Identification of a potent and highly efficacious, yet slowly desensitizing CB1 cannabinoid receptor agonist. Br J Pharmacol. 2004;142(3):495–500. https://doi.org/10.1038/sj.bjp.0705792.
Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000;57(5):1045–50.
Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther. 2000;295(1):328–36.
Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578(Pt 1):233–47. https://doi.org/10.1113/jphysiol.2006.115691.
Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25(42):9746–51. https://doi.org/10.1523/JNEUROSCI.2769-05.2005.
Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, et al. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45(2):257–68. https://doi.org/10.1016/j.neuron.2005.01.004.
Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26(16):4166–77. https://doi.org/10.1523/JNEUROSCI.0176-06.2006.
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–7. https://doi.org/10.1038/384083a0.
Woodward DF, Liang Y, Krauss AH. Prostamides (prostaglandin-ethanolamides) and their pharmacology. Br J Pharmacol. 2008;153(3):410–9. https://doi.org/10.1038/sj.bjp.0707434.
Hermanson DJ, Hartley ND, Gamble-George J, Brown N, Shonesy BC, Kingsley PJ, et al. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat Neurosci. 2013;16(9):1291–8. https://doi.org/10.1038/nn.3480.
Hermanson DJ, Gamble-George JC, Marnett LJ, Patel S. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci. 2014;35(7):358–67. https://doi.org/10.1016/j.tips.2014.04.006.
Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14(12):1347–56. https://doi.org/10.1016/j.chembiol.2007.11.006.
Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7(7):697–8. https://doi.org/10.1038/nn1262.
Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334(6057):809–13. https://doi.org/10.1126/science.1209200.
Howlett AC, Abood ME. CB1 and CB2 receptor pharmacology. Adv Pharmacol (San Diego, Calif). 2017;80:169–206. https://doi.org/10.1016/bs.apha.2017.03.007.
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–4. https://doi.org/10.1038/346561a0.
Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–5. https://doi.org/10.1038/365061a0.
Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15(10):6552–61.
Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78(1):43–50. https://doi.org/10.1152/jn.1997.78.1.43.
Onaivi ES, Ishiguro H, Gu S, Liu QR. CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol. 2012;26(1):92–103. https://doi.org/10.1177/0269881111400652.
Navarrete F, Perez-Ortiz JM, Manzanares J. Cannabinoid CB(2) receptor-mediated regulation of impulsive-like behaviour in DBA/2 mice. Br J Pharmacol. 2012;165(1):260–73. https://doi.org/10.1111/j.1476-5381.2011.01542.x.
Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br J Pharmacol. 2012;165(4):951–64. https://doi.org/10.1111/j.1476-5381.2011.01625.x.
Chen DJ, Gao M, Gao FF, Su QX, Wu J. Brain cannabinoid receptor 2: expression, function and modulation. Acta Pharmacol Sin. 2017;38(3):312–6. https://doi.org/10.1038/aps.2016.149.
Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, et al. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron. 2016;90(4):795–809. https://doi.org/10.1016/j.neuron.2016.03.034.
Li Y, Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015;311:253–67. https://doi.org/10.1016/j.neuroscience.2015.10.041.
Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, et al. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci. 2009;29(14):4564–70. https://doi.org/10.1523/JNEUROSCI.0786-09.2009.
Bradshaw HB, Walker JM. The expanding field of cannabimimetic and related lipid mediators. Br J Pharmacol. 2005;144(4):459–65. https://doi.org/10.1038/sj.bjp.0706093.
Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, et al. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993;214(1):173–80. https://doi.org/10.1111/j.1432-1033.1993.tb17910.x.
Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23(4):1398–405.
Ramirez SH, Hasko J, Skuba A, Fan S, Dykstra H, McCormick R, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32(12):4004–16. https://doi.org/10.1523/jneurosci.4628-11.2012.
Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A. 2014;111(46):E5007–15. https://doi.org/10.1073/pnas.1413210111.
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–7. https://doi.org/10.1038/22761.
Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517(3):174–81. https://doi.org/10.1016/j.ejphar.2005.05.032.
Taylor SJ, Chae HZ, Rhee SG, Exton JH. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991;350(6318):516–8. https://doi.org/10.1038/350516a0.
Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89(1):309–80. https://doi.org/10.1152/physrev.00019.2008.
Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99(12):8384–8. https://doi.org/10.1073/pnas.122149199.
Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5(5):446–51. https://doi.org/10.1038/nn832.
Tsetsenis T, Younts TJ, Chiu CQ, Kaeser PS, Castillo PE, Sudhof TC. Rab3B protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning. Proc Natl Acad Sci U S A. 2011;108(34):14300–5. https://doi.org/10.1073/pnas.1112237108.
Jiang B, Huang S, de Pasquale R, Millman D, Song L, Lee HK, et al. The maturation of GABAergic transmission in visual cortex requires endocannabinoid-mediated LTD of inhibitory inputs during a critical period. Neuron. 2010;66(2):248–59. https://doi.org/10.1016/j.neuron.2010.03.021.
Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54(5):801–12. https://doi.org/10.1016/j.neuron.2007.05.020.
Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43(6):871–81. https://doi.org/10.1016/j.neuron.2004.08.036.
Marinelli S, Pacioni S, Cannich A, Marsicano G, Bacci A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009;12(12):1488–90. https://doi.org/10.1038/nn.2430.
Marinelli S, Pacioni S, Bisogno T, Di Marzo V, Prince DA, Huguenard JR, et al. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J Neurosci. 2008;28(50):13532–41. https://doi.org/10.1523/JNEUROSCI.0847-08.2008.
Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431(7006):312–6. https://doi.org/10.1038/nature02913.
Dubreucq S, Durand A, Matias I, Benard G, Richard E, Soria-Gomez E, et al. Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biol Psychiatry. 2013;73(9):895–903. https://doi.org/10.1016/j.biopsych.2012.10.025.
Albayram O, Passlick S, Bilkei-Gorzo A, Zimmer A, Steinhauser C. Physiological impact of CB1 receptor expression by hippocampal GABAergic interneurons. Pflugers Arch. 2016;468(4):727–37. https://doi.org/10.1007/s00424-015-1782-5.
Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–32. https://doi.org/10.1126/science.1115740.
Hillard CJ, Beatka M, Sarvaideo J. Endocannabinoid signaling and the hypothalamic-pituitary-adrenal axis. Compr Physiol. 2016;7(1):1–15. https://doi.org/10.1002/cphy.c160005.
Hedrich J, Angamo EA, Conrad A, Lutz B, Luhmann HJ. Cell type specific impact of cannabinoid receptor signaling in somatosensory barrel map formation in mice. J Comp Neurol. 2019. https://doi.org/10.1002/cne.24733.
Friend LN, Williamson RC, Merrill CB, Newton ST, Christensen MT, Petersen J, et al. Hippocampal stratum oriens somatostatin-positive cells undergo CB1-dependent long-term potentiation and express endocannabinoid biosynthetic enzymes. Molecules. 2019;24(7). https://doi.org/10.3390/molecules24071306.
Fernandez-Espejo E, Nunez-Dominguez L. Endocannabinoid-mediated synaptic plasticity and substance use disorders. Neurologia. 2019. https://doi.org/10.1016/j.nrl.2018.12.004.
Dow-Edwards D, Silva L. Endocannabinoids in brain plasticity: cortical maturation, HPA axis function and behavior. Brain Res. 2017;1654(Pt B):157–64. https://doi.org/10.1016/j.brainres.2016.08.037.
Augustin SM, Lovinger DM. Functional relevance of endocannabinoid-dependent synaptic plasticity in the central nervous system. ACS Chem Neurosci. 2018;9(9):2146–61. https://doi.org/10.1021/acschemneuro.7b00508.
Guo S, Liu Y, Ma R, Li J, Su B. Neuroprotective effect of endogenous cannabinoids on ischemic brain injury induced by the excess microglia-mediated inflammation. Am J Transl Res. 2016;8(6):2631–40.
Szczesniak AM, Porter RF, Toguri JT, Borowska-Fielding J, Gebremeskel S, Siwakoti A, et al. Cannabinoid 2 receptor is a novel anti-inflammatory target in experimental proliferative vitreoretinopathy. Neuropharmacology. 2017;113(Pt B):627–38. https://doi.org/10.1016/j.neuropharm.2016.08.030.
Zurier RB, Burstein SH. Cannabinoids, inflammation, and fibrosis. FASEB J. 2016;30(11):3682–9. https://doi.org/10.1096/fj.201600646R.
Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56(25):2115–25. https://doi.org/10.1016/j.jacc.2010.07.033.
Benito C, Romero JP, Tolon RM, Clemente D, Docagne F, Hillard CJ, et al. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci. 2007;27(9):2396–402. https://doi.org/10.1523/jneurosci.4814-06.2007.
Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25(8):1904–13. https://doi.org/10.1523/jneurosci.4540-04.2005.
Mbvundula EC, Bunning RA, Rainsford KD. Arthritis and cannabinoids: HU-210 and Win-55,212-2 prevent IL-1alpha-induced matrix degradation in bovine articular chondrocytes in-vitro. J Pharm Pharmacol. 2006;58(3):351–8. https://doi.org/10.1211/jpp.58.3.0009.
Tomar S, Zumbrun EE, Nagarkatti M, Nagarkatti PS. Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs. J Pharmacol Exp Ther. 2015;353(2):369–79. https://doi.org/10.1124/jpet.114.220368.
Liu Y, Fassbender K. Deficiency of TLR4 ameliorates hypoperfusion-induced brain pathology. Theranostics. 2018;8(22):6355–6. https://doi.org/10.7150/thno.30953.
Lee W, Park JY, Chun YM. Operative treatment of 2-part surgical neck fracture of the humerus: intramedullary nail versus locking compression plate with technical consideration. J Orthop Trauma. 2017;31(9):e270–e4. https://doi.org/10.1097/BOT.0000000000000916.
Kim MS, Bang JH, Lee J, Kim HW, Sung SH, Han JS, et al. Salvia miltiorrhiza extract protects white matter and the hippocampus from damage induced by chronic cerebral hypoperfusion in rats. BMC Complement Altern Med. 2015;15:415. https://doi.org/10.1186/s12906-015-0943-6.
Lee KM, Bang J, Kim BY, Lee IS, Han JS, Hwang BY, et al. Fructus mume alleviates chronic cerebral hypoperfusion-induced white matter and hippocampal damage via inhibition of inflammation and downregulation of TLR4 and p38 MAPK signaling. BMC Complement Altern Med. 2015;15:125. https://doi.org/10.1186/s12906-015-0652-1.
Srivastava T, Diba P, Dean JM, Banine F, Shaver D, Hagen M, et al. A TLR/AKT/FoxO3 immune tolerance-like pathway disrupts the repair capacity of oligodendrocyte progenitors. J Clin Invest. 2018;128(5):2025–41. https://doi.org/10.1172/jci94158.
Habib A, Chokr D, Wan J, Hegde P, Mabire M, Siebert M, et al. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut. 2018. https://doi.org/10.1136/gutjnl-2018-316137.
Xiang W, Shi R, Kang X, Zhang X, Chen P, Zhang L, et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat Commun. 2018;9(1):2574. https://doi.org/10.1038/s41467-018-04999-8.
Tobin RP, Mukherjee S, Kain JM, Rogers SK, Henderson SK, Motal HL, et al. Traumatic brain injury causes selective, CD74-dependent peripheral lymphocyte activation that exacerbates neurodegeneration. Acta Neuropathol Commun. 2014;2(1):143. https://doi.org/10.1186/s40478-014-0143-5.
Hua R, Mao SS, Zhang YM, Chen FX, Zhou ZH, Liu JQ. Effects of pituitary adenylate cyclase activating polypeptide on CD4(+)/CD8(+) T cell levels after traumatic brain injury in a rat model. World J Emerg Med. 2012;3(4):294–8. https://doi.org/10.5847/wjem.j.1920-8642.2012.04.010.
Amick JE, Yandora KA, Bell MJ, Wisniewski SR, Adelson PD, Carcillo JA, et al. The Th1 versus Th2 cytokine profile in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Crit Care Med. 2001;2(3):260–4.
Ojo JO, Greenberg MB, Leary P, Mouzon B, Bachmeier C, Mullan M, et al. Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Front Behav Neurosci. 2014;8:213. https://doi.org/10.3389/fnbeh.2014.00213.
Braun M, Vaibhav K, Saad N, Fatima S, Brann DW, Vender JR, et al. Activation of myeloid TLR4 mediates t lymphocyte polarization after traumatic brain injury. J Immunol. 2017;198(9):3615–26. https://doi.org/10.4049/jimmunol.1601948.
Braun M, Vaibhav K, Saad NM, Fatima S, Vender JR, Baban B, et al. White matter damage after traumatic brain injury: a role for damage associated molecular patterns. Biochim Biophys Acta Mol basis Dis. 2017;1863(10 Pt B):2614–26. https://doi.org/10.1016/j.bbadis.2017.05.020.
Alberti TB, Barbosa WL, Vieira JL, Raposo NR, Dutra RC. (-)-Beta-caryophyllene, a CB2 receptor-selective phytocannabinoid, suppresses motor paralysis and neuroinflammation in a murine model of multiple sclerosis. Int J Mol Sci. 2017;18(4). https://doi.org/10.3390/ijms18040691.
Annunziata P, Cioni C, Mugnaini C, Corelli F. Potent immunomodulatory activity of a highly selective cannabinoid CB2 agonist on immune cells from healthy subjects and patients with multiple sclerosis. J Neuroimmunol. 2017;303:66–74. https://doi.org/10.1016/j.jneuroim.2016.12.009.
Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78(16):1665–703. https://doi.org/10.1007/s40265-018-0996-1.
Schurman LD, Lichtman AH. Endocannabinoids: a promising impact for traumatic brain injury. Front Pharmacol. 2017;8:69. https://doi.org/10.3389/fphar.2017.00069.
Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, et al. Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg. 1998;89(4):507–18. https://doi.org/10.3171/jns.1998.89.4.0507.
Zhao J, Moore AN, Clifton GL, Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005;82(4):499–506. https://doi.org/10.1002/jnr.20649.
Meyer MJ, Megyesi J, Meythaler J, Murie-Fernandez M, Aubut JA, Foley N, et al. Acute management of acquired brain injury part II: an evidence-based review of pharmacological interventions. Brain Inj. 2010;24(5):706–21.
Donat CK, Fischer F, Walter B, Deuther-Conrad W, Brodhun M, Bauer R, et al. Early increase of cannabinoid receptor density after experimental traumatic brain injury in the newborn piglet. Acta Neurobiol Exp. 2014;74(2):197–210.
Amenta PS, Jallo JI, Tuma RF, Elliott MB. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J Neurosci Res. 2012;90(12):2293–305. https://doi.org/10.1002/jnr.23114.
Shohami E, Cohen-Yeshurun A, Magid L, Algali M, Mechoulam R. Endocannabinoids and traumatic brain injury. Br J Pharmacol. 2011;163(7):1402–10. https://doi.org/10.1111/j.1476-5381.2011.01343.x.
Xing G, Carlton J, Jiang X, Wen J, Jia M, Li H. Differential expression of brain cannabinoid receptors between repeatedly stressed males and females may play a role in age and gender-related difference in traumatic brain injury: implications from animal studies. Front Neurol. 2014;5:161. https://doi.org/10.3389/fneur.2014.00161.
Mechoulam R, Shohami E. Endocannabinoids and traumatic brain injury. Mol Neurobiol. 2007;36(1):68–74. https://doi.org/10.1007/s12035-007-8008-6.
Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, et al. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22(2):257–64. https://doi.org/10.1016/j.nbd.2005.11.004.
Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J Cereb Blood Flow Metab. 2005;25(4):477–84. https://doi.org/10.1038/sj.jcbfm.9600047.
Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413(6855):527–31. https://doi.org/10.1038/35097089.
Mayeux J, Katz P, Edwards S, Middleton JW, Molina PE. Inhibition of endocannabinoid degradation improves outcomes from mild traumatic brain injury: a mechanistic role for synaptic hyperexcitability. J Neurotrauma. 2017;34(2):436–43. https://doi.org/10.1089/neu.2016.4452.
Tchantchou F, Zhang Y. Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J Neurotrauma. 2013;30(7):565–79. https://doi.org/10.1089/neu.2012.2647.
Selvaraj P, Wen J, Tanaka M, Zhang Y. Therapeutic effect of a novel fatty acid amide hydrolase inhibitor PF04457845 in the repetitive closed head injury mouse model. J Neurotrauma. 2019;36(10):1655–69. https://doi.org/10.1089/neu.2018.6226.
Katz PS, Sulzer JK, Impastato RA, Teng SX, Rogers EK, Molina PE. Endocannabinoid degradation inhibition improves neurobehavioral function, blood-brain barrier integrity, and neuroinflammation following mild traumatic brain injury. J Neurotrauma. 2015;32(5):297–306. https://doi.org/10.1089/neu.2014.3508.
Tchantchou F, Tucker LB, Fu AH, Bluett RJ, McCabe JT, Patel S, et al. The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology. 2014;85:427–39. https://doi.org/10.1016/j.neuropharm.2014.06.006.
Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, et al. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep. 2012;2(5):1329–39. https://doi.org/10.1016/j.celrep.2012.09.030.
Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-β pathology: a link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11(5):361–70. https://doi.org/10.1038/nrn2808.
Goldstein LE, Fisher AM, Tagge CA, Zhang X-L, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra60-ra60. https://doi.org/10.1126/scitranslmed.3003716.
Smith DH, Chen XH, Nonaka M, Trojanowski JQ, Lee VM, Saatman KE, et al. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999;58(9):982–92. https://doi.org/10.1097/00005072-199909000-00008.
Amenta PS, Jallo JI, Tuma RF, Elliott MB. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J Neurosci Res. 2012;90(12):2293–305. https://doi.org/10.1002/jnr.23114.
Schmitt U, Tanimoto N, Seeliger M, Schaeffel F, Leube RE. Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience. 2009;162(2):234–43. https://doi.org/10.1016/j.neuroscience.2009.04.046.
Hind WH, Tufarelli C, Neophytou M, Anderson SI, England TJ, O'Sullivan SE. Endocannabinoids modulate human blood-brain barrier permeability in vitro. Br J Pharmacol. 2015;172(12):3015–27. https://doi.org/10.1111/bph.13106.
Hu D-E, Easton AS, Fraser PA. TRPV1 activation results in disruption of the blood-brain barrier in the rat. Br J Pharmacol. 2005;146(4):576–84. https://doi.org/10.1038/sj.bjp.0706350.
Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fat Acids. 2002;66(2–3):101–21. https://doi.org/10.1054/plef.2001.0341.
Guley NM, Del Mar NA, Ragsdale T, Li C, Perry AM, Moore BM, et al. Amelioration of visual deficits and visual system pathology after mild TBI with the cannabinoid type-2 receptor inverse agonist SMM-189. Exp Eye Res. 2019;182:109–24. https://doi.org/10.1016/j.exer.2019.03.013.
Honig MG, Del Mar NA, Henderson DL, Ragsdale TD, Doty JB, Driver JH, et al. Amelioration of visual deficits and visual system pathology after mild TBI via the cannabinoid type-2 receptor inverse agonism of raloxifene. Exp Neurol. 2019;322:113063. https://doi.org/10.1016/j.expneurol.2019.113063.
Lopez-Rodriguez AB, Mela V, Acaz-Fonseca E, Garcia-Segura LM, Viveros M-P. CB2 cannabinoid receptor is involved in the anti-inflammatory effects of leptin in a model of traumatic brain injury. Exp Neurol. 2016;279:274–82. https://doi.org/10.1016/j.expneurol.2016.03.018.
Lopez-Rodriguez AB, Siopi E, Finn DP, Marchand-Leroux C, Garcia-Segura LM, Jafarian-Tehrani M, et al. CB1 and CB2 cannabinoid receptor antagonists prevent minocycline-induced neuroprotection following traumatic brain injury in mice. Cereb Cortex. 2015;25(1):35–45. https://doi.org/10.1093/cercor/bht202.
López Rodríguez AB, Mateos Vicente B, Romero-Zerbo SY, Rodriguez-Rodriguez N, Bellini MJ, Rodriguez de Fonseca F, et al. Estradiol decreases cortical reactive astrogliosis after brain injury by a mechanism involving cannabinoid receptors. Cereb Cortex. 2011;21(9):2046–55. https://doi.org/10.1093/cercor/bhq277.
Walker R, Cole JE, Logan TK, Corrigan JD. Screening substance abuse treatment clients for traumatic brain injury: prevalence and characteristics. J Head Trauma Rehabil. 2007;22(6):360–7. https://doi.org/10.1097/01.htr.0000300231.90619.50.
O'Phelan K, McArthur DL, Chang CW, Green D, Hovda DA. The impact of substance abuse on mortality in patients with severe traumatic brain injury. J Trauma. 2008;65(3):674–7. https://doi.org/10.1097/TA.0b013e31817db0a5.
Hawley LA, Ketchum JM, Morey C, Collins K, Charlifue S. Cannabis use in individuals with spinal cord injury or moderate to severe traumatic brain injury in Colorado. Arch Phys Med Rehabil. 2018;99(8):1584–90. https://doi.org/10.1016/j.apmr.2018.02.003.
Nguyen BM, Kim D, Bricker S, Bongard F, Neville A, Putnam B, et al. Effect of marijuana use on outcomes in traumatic brain injury. Am Surg. 2014;80(10):979–83.
Lawrence DW, Foster E, Comper P, Langer L, Hutchison MG, Chandra T, et al. Cannabis, alcohol and cigarette use during the acute post-concussion period. Brain Inj. 2020;34(1):42–51. https://doi.org/10.1080/02699052.2019.1679885.
Grenier K, Ponnambalam F, Lee D, Lauwers R, Bhalerao S. Cannabis in the treatment of traumatic brain injury: a primer for clinicians. Can J Neurol Sci. 2019:1–7. https://doi.org/10.1017/cjn.2019.298.
Rabner J, Gottlieb S, Lazdowsky L, LeBel A. Psychosis following traumatic brain injury and cannabis use in late adolescence. Am J Addict. 2016;25(2):91–3. https://doi.org/10.1111/ajad.12338.
Polivka J Jr, Polivka J, Pesta M, Rohan V, Celedova L, Mahajani S, et al. Risks associated with the stroke predisposition at young age: facts and hypotheses in light of individualized predictive and preventive approach. EPMA J. 2019;10(1):81–99. https://doi.org/10.1007/s13167-019-00162-5.
England TJ, Hind WH, Rasid NA, O'Sullivan SE. Cannabinoids in experimental stroke: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2015;35(3):348–58. https://doi.org/10.1038/jcbfm.2014.218.
Suzuki N, Suzuki M, Murakami K, Hamajo K, Tsukamoto T, Shimojo M. Cerebroprotective effects of TAK-937, a cannabinoid receptor agonist, on ischemic brain damage in middle cerebral artery occluded rats and non-human primates. Brain Res. 2012;1430:93–100. https://doi.org/10.1016/j.brainres.2011.10.044.
Leker RR, Gai N, Mechoulam R, Ovadia H. Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU-210. Stroke. 2003;34(8):2000–6. https://doi.org/10.1161/01.STR.0000079817.68944.1E.
Bravo-Ferrer I, Cuartero MI, Zarruk JG, Pradillo JM, Hurtado O, Romera VG, et al. Cannabinoid type-2 receptor drives neurogenesis and improves functional outcome after stroke. Stroke. 2017;48(1):204–12. https://doi.org/10.1161/STROKEAHA.116.014793.
Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J, et al. Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke. 2009;40(6):2157–64. https://doi.org/10.1161/STROKEAHA.108.541490.
Ceprián M, Jiménez-Sánchez L, Vargas C, Barata L, Hind W, Martínez-Orgado J. Cannabidiol reduces brain damage and improves functional recovery in a neonatal rat model of arterial ischemic stroke. Neuropharmacology. 2017;116:151–9. https://doi.org/10.1016/j.neuropharm.2016.12.017.
Hind WH, England TJ, O’Sullivan SE. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br J Pharmacol. 2016;173(5):815–25. https://doi.org/10.1111/bph.13368.
Rajesh M, Mukhopadhyay P, Bátkai S, Haskó G, Liaudet L, Drel VR, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. 2007;293(1):H610–H9. https://doi.org/10.1152/ajpheart.00236.2007.
Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke. 2005;36(5):1077–82. https://doi.org/10.1161/01.STR.0000163083.59201.34.
Alvarez FJ, Lafuente H, Rey-Santano MC, Mielgo VE, Gastiasoro E, Rueda M, et al. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr Res. 2008;64(6):653–8. https://doi.org/10.1203/PDR.0b013e318186e5dd.
Sultan SR, Millar SA, England TJ, O’Sullivan SE. A systematic review and meta-analysis of the haemodynamic effects of cannabidiol. Front Pharmacol. 2017;8:81. https://doi.org/10.3389/fphar.2017.00081.
Jadoon KA, Tan GD, O'Sullivan SE. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight. 2017;2(12):e93760. https://doi.org/10.1172/jci.insight.93760.
Hayakawa K, Irie K, Sano K, Watanabe T, Higuchi S, Enoki M, et al. Therapeutic time window of cannabidiol treatment on delayed ischemic damage via high-mobility group box1-inhibiting mechanism. Biol Pharm Bull. 2009;32(9):1538–44. https://doi.org/10.1248/bpb.32.1538.
Hayakawa K, Mishima K, Fujiwara M. Therapeutic potential of non-psychotropic cannabidiol in ischemic stroke. Pharmaceuticals (Basel, Switzerland). 2010;3(7):2197–212. https://doi.org/10.3390/ph3072197.
Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci. 2002;22(22):9771–5. https://doi.org/10.1523/JNEUROSCI.22-22-09771.2002.
Hayakawa K, Mishima K, Nozako M, Hazekawa M, Ogata A, Fujioka M, et al. Delta9-tetrahydrocannabinol (delta9-THC) prevents cerebral infarction via hypothalamic-independent hypothermia. Life Sci. 2007;80(16):1466–71. https://doi.org/10.1016/j.lfs.2007.01.014.
Choi S-H, Mou Y, Silva AC. Cannabis and cannabinoid biology in stroke. Stroke. 2019;50(9):2640–5. https://doi.org/10.1161/STROKEAHA.118.023587.
Wolff V, Jouanjus E. Strokes are possible complications of cannabinoids use. Epilepsy Behav. 2017;70(Pt B):355–63. https://doi.org/10.1016/j.yebeh.2017.01.031.
Shearer JA, Coker SJ, Carswell HVO. Detrimental effects of 2-arachidonoylglycerol on whole blood platelet aggregation and on cerebral blood flow after a focal ischemic insult in rats. Am J Physiol Heart Circ Physiol. 2018;314(5):H967–H77. https://doi.org/10.1152/ajpheart.00299.2017.
Keown OP, Winterburn TJ, Wainwright CL, Macrury SM, Neilson I, Barrett F, et al. 2-Arachidonyl glycerol activates platelets via conversion to arachidonic acid and not by direct activation of cannabinoid receptors. Br J Clin Pharmacol. 2010;70(2):180–8. https://doi.org/10.1111/j.1365-2125.2010.03697.x.
Brantl SA, Khandoga AL, Siess W. Mechanism of platelet activation induced by endocannabinoids in blood and plasma. Platelets. 2014;25(3):151–61. https://doi.org/10.3109/09537104.2013.803530.
Polivka J, Polivka J Jr, Rohan V. Predictive and individualized management of stroke-success story in Czech Republic. EPMA J. 2018;9(4):393–401. https://doi.org/10.1007/s13167-018-0150-x.
Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5:40. https://doi.org/10.1186/1750-1172-5-40.
Pazos MR, Sagredo O, Fernandez-Ruiz J. The endocannabinoid system in Huntington’s disease. Curr Pharm Des. 2008;14(23):2317–25. https://doi.org/10.2174/138161208785740108.
Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease—successes and failures. FEBS J. 2013;280(9):1918–43. https://doi.org/10.1111/febs.12260.
Micale V, Mazzola C, Drago F. Endocannabinoids and neurodegenerative diseases. Pharmacol Res. 2007;56(5):382–92. https://doi.org/10.1016/j.phrs.2007.09.008.
Maccarrone M, Battista N, Centonze D. The endocannabinoid pathway in Huntington's disease: a comparison with other neurodegenerative diseases. Prog Neurobiol. 2007;81(5–6):349–79. https://doi.org/10.1016/j.pneurobio.2006.11.006.
Lastres-Becker I, Bizat N, Boyer F, Hantraye P, Brouillet E, Fernandez-Ruiz J. Effects of cannabinoids in the rat model of Huntington’s disease generated by an intrastriatal injection of malonate. Neuroreport. 2003;14(6):813–6. https://doi.org/10.1097/00001756-200305060-00007.
Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445(7128):643–7. https://doi.org/10.1038/nature05506.
Dowie MJ, Bradshaw HB, Howard ML, Nicholson LF, Faull RL, Hannan AJ, et al. Altered CB1 receptor and endocannabinoid levels precede motor symptom onset in a transgenic mouse model of Huntington’s disease. Neuroscience. 2009;163(1):456–65. https://doi.org/10.1016/j.neuroscience.2009.06.014.
Bisogno T, Di Marzo V. The role of the endocannabinoid system in Alzheimer’s disease: facts and hypotheses. Curr Pharm Des. 2008;14(23):2299–3305. https://doi.org/10.2174/138161208785740027.
Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3(9):771–84. https://doi.org/10.1038/nrd1495.
Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain. 2009;132(Pt 11):3152–64. https://doi.org/10.1093/brain/awp239.
Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87(3):493–506. https://doi.org/10.1016/s0092-8674(00)81369-0.
Sapp E, Kegel KB, Aronin N, Hashikawa T, Uchiyama Y, Tohyama K, et al. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol Exp Neurol. 2001;60(2):161–72. https://doi.org/10.1093/jnen/60.2.161.
Sagredo O, Pazos MR, Valdeolivas S, Fernandez-Ruiz J. Cannabinoids: novel medicines for the treatment of Huntington’s disease. Recent Pat CNS Drug Discov. 2012;7(1):41–8. https://doi.org/10.2174/157488912798842278.
Valdeolivas S, Navarrete C, Cantarero I, Bellido ML, Muñoz E, Sagredo O. Neuroprotective properties of cannabigerol in Huntington’s disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics. 2015;12(1):185–99. https://doi.org/10.1007/s13311-014-0304-z.
Díaz-Alonso J, Paraíso-Luna J, Navarrete C, Del Río C, Cantarero I, Palomares B, et al. VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease. Sci Rep. 2016;6:29789. https://doi.org/10.1038/srep29789.
Saft C, von Hein SM, Lücke T, Thiels C, Peball M, Djamshidian A, et al. Cannabinoids for treatment of dystonia in Huntington’s disease. J Huntingtons Dis. 2018;7(2):167–73. https://doi.org/10.3233/JHD-170283.
Zarei S, Carr K, Reiley L, Diaz K, Guerra O, Altamirano PF, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015;6:171. https://doi.org/10.4103/2152-7806.169561.
Flavell L. Approved treatments. ALS News Today 2020. https://alsnewstoday.com/approved-treatments/. Accessed January 9 2020.
FDA-approved drugs. The ALS Association. 2020. http://www.alsa.org/als-care/fda-approved-drugs.html. Accessed January 9 2020.
Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7(11):639–49. https://doi.org/10.1038/nrneurol.2011.153.
Matamala JM, Dharmadasa T, Kiernan MC. Prognostic factors in C9orf72 amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(4):281. https://doi.org/10.1136/jnnp-2016-314685.
Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2007;(1):CD001447-CD. https://doi.org/10.1002/14651858.CD001447.pub2.
Raman C, McAllister SD, Rizvi G, Patel SG, Moore DH, Abood ME. Amyotrophic lateral sclerosis: delayed disease progression in mice by treatment with a cannabinoid. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(1):33–9. https://doi.org/10.1080/14660820310016813.
Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J, de Lago E. Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a Sativex(®)-like combination of phytocannabinoids: interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther. 2014;20(9):809–15. https://doi.org/10.1111/cns.12262.
Weydt P, Hong S, Witting A, Möller T, Stella N, Kliot M. Cannabinol delays symptom onset in SOD1 (G93A) transgenic mice without affecting survival. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(3):182–4. https://doi.org/10.1080/14660820510030149.
Bilsland LG, Dick JRT, Pryce G, Petrosino S, Di Marzo V, Baker D, et al. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J. 2006;20(7):1003–5. https://doi.org/10.1096/fj.05-4743fje.
Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. https://doi.org/10.1186/1471-2377-6-12.
Shoemaker JL, Seely KA, Reed RL, Crow JP, Prather PL. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem. 2007;101(1):87–98. https://doi.org/10.1111/j.1471-4159.2006.04346.x.
Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141(5):775–85. https://doi.org/10.1038/sj.bjp.0705667.
Amtmann D, Weydt P, Johnson KL, Jensen MP, Carter GT. Survey of cannabis use in patients with amyotrophic lateral sclerosis. Am J Hosp Palliat Care. 2004;21(2):95–104. https://doi.org/10.1177/104990910402100206.
Carter GT, Rosen BS. Marijuana in the management of amyotrophic lateral sclerosis. Am J Hosp Palliat Care. 2001;18(4):264–70. https://doi.org/10.1177/104990910101800411.
Urbi B, Broadley S, Bedlack R, Russo E, Sabet A. Study protocol for a randomised, double-blind, placebo-controlled study evaluating the Efficacy of cannabis-based Medicine Extract in slowing the disease pRogression of Amyotrophic Lateral sclerosis or motor neurone Disease: the EMERALD trial. BMJ Open. 2019;9(11):e029449-e. https://doi.org/10.1136/bmjopen-2019-029449.
Baul HS, Manikandan C, Sen D. Cannabinoid receptor as a potential therapeutic target for Parkinson’s disease. Brain Res Bull. 2019;146:244–52. https://doi.org/10.1016/j.brainresbull.2019.01.016.
Ceccarini J, Casteels C, Ahmad R, Crabbé M, Van de Vliet L, Vanhaute H, et al. Regional changes in the type 1 cannabinoid receptor are associated with cognitive dysfunction in Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2019;46(11):2348–57. https://doi.org/10.1007/s00259-019-04445-x.
Meireles J, Massano J. Cognitive impairment and dementia in parkinson’s disease: clinical features, diagnosis, and management. Front Neurol. 2012;3(88). https://doi.org/10.3389/fneur.2012.00088.
Tang Y, Ge J, Liu F, Wu P, Guo S, Liu Z, et al. Cerebral metabolic differences associated with cognitive impairment in Parkinson’s disease. PLoS One. 2016;11(4):e0152716. https://doi.org/10.1371/journal.pone.0152716.
Broeders M, Velseboer DC, de Bie R, Speelman JD, Muslimovic D, Post B, et al. Cognitive change in newly-diagnosed patients with Parkinson’s disease: a 5-year follow-up study. J Int Neuropsychol Soc. 2013;19(6):695–708. https://doi.org/10.1017/S1355617713000295.
MuslimoviĆ D, Post B, Speelman JD, De Haan RJ, Schmand BEN. Cognitive decline in Parkinson’s disease: a prospective longitudinal study. J Int Neuropsychol Soc. 2009;15(3):426–37. https://doi.org/10.1017/S1355617709090614.
Han Q-W, Yuan Y-H, Chen N-H. The therapeutic role of cannabinoid receptors and its agonists or antagonists in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109745. https://doi.org/10.1016/j.pnpbp.2019.109745.
van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, et al. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J. 2005;19(9):1140–2. https://doi.org/10.1096/fj.04-3010fje.
Segovia G, Mora F, Crossman AR, Brotchie JM. Effects of CB1 cannabinoid receptor modulating compounds on the hyperkinesia induced by high-dose levodopa in the reserpine-treated rat model of Parkinson’s disease. Mov Disord. 2003;18(2):138–49. https://doi.org/10.1002/mds.10312.
Scotter EL, Abood ME, Glass M. The endocannabinoid system as a target for the treatment of neurodegenerative disease. Br J Pharmacol. 2010;160(3):480–98. https://doi.org/10.1111/j.1476-5381.2010.00735.x.
Pisani V, Madeo G, Tassone A, Sciamanna G, Maccarrone M, Stanzione P, et al. Homeostatic changes of the endocannabinoid system in Parkinson’s disease. Mov Disord. 2011;26(2):216–22. https://doi.org/10.1002/mds.23457.
Pisani A, Fezza F, Galati S, Battista N, Napolitano S, Finazzi-Agro A, et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann Neurol. 2005;57(5):777–9. https://doi.org/10.1002/ana.20462.
Di Filippo M, Picconi B, Tozzi A, Ghiglieri V, Rossi A, Calabresi P. The endocannabinoid system in Parkinson’s disease. Curr Pharm Des. 2008;14(23):2337–47. https://doi.org/10.2174/138161208785740072.
Centonze D, Finazzi-Agro A, Bernardi G, Maccarrone M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol Sci. 2007;28(4):180–7. https://doi.org/10.1016/j.tips.2007.02.004.
Brotchie JM. CB1 cannabinoid receptor signalling in Parkinson’s disease. Curr Opin Pharmacol. 2003;3(1):54–61.
Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18(6):1607–14. https://doi.org/10.1046/j.1460-9568.2003.02896.x.
Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, et al. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22(16):6900–7. https://doi.org/10.1523/jneurosci.22-16-06900.2002.
Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109(3):451–60. https://doi.org/10.1016/s0306-4522(01)00509-7.
Navarrete F, García-Gutiérrez MS, Aracil-Fernández A, Lanciego JL, Manzanares J. Cannabinoid CB1 and CB2 receptors, and monoacylglycerol lipase gene expression alterations in the basal ganglia of patients with Parkinson’s disease. Neurotherapeutics. 2018;15(2):459–69. https://doi.org/10.1007/s13311-018-0603-x.
Grunblatt E, Zander N, Bartl J, Jie L, Monoranu CM, Arzberger T, et al. Comparison analysis of gene expression patterns between sporadic Alzheimer’s and Parkinson’s disease. J Alzheimers Dis. 2007;12(4):291–311. https://doi.org/10.3233/jad-2007-12402.
Sierra S, Luquin N, Rico AJ, Gómez-Bautista V, Roda E, Dopeso-Reyes IG, et al. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct Funct. 2015;220(5):2721–38. https://doi.org/10.1007/s00429-014-0823-8.
Navarro G, Borroto-Escuela D, Angelats E, Etayo Í, Reyes-Resina I, Pulido-Salgado M, et al. Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB1 and CB2 receptors and relevance for Alzheimer’s disease and levodopa-induced dyskinesia. Brain Behav Immun. 2018;67:139–51. https://doi.org/10.1016/j.bbi.2017.08.015.
Pisani V, Moschella V, Bari M, Fezza F, Galati S, Bernardi G, et al. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov Disord. 2010;25(7):920–4. https://doi.org/10.1002/mds.23014.
Maccarrone M, Gubellini P, Bari M, Picconi B, Battista N, Centonze D, et al. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J Neurochem. 2003;85(4):1018–25. https://doi.org/10.1046/j.1471-4159.2003.01759.x.
Celorrio M, Fernández-Suárez D, Rojo-Bustamante E, Echeverry-Alzate V, Ramírez MJ, Hillard CJ, et al. Fatty acid amide hydrolase inhibition for the symptomatic relief of Parkinson’s disease. Brain Behav Immun. 2016;57:94–105. https://doi.org/10.1016/j.bbi.2016.06.010.
Viveros-Paredes JM, Gonzalez-Castañeda RE, Escalante-Castañeda A, Tejeda-Martínez AR, Castañeda-Achutiguí F, Flores-Soto ME. Efecto del inhibidor de amida hidrolasa de ácidos grasos en el daño neuronal dopaminérgico inducido por MPTP. Neurología. 2019;34(3):143–52. https://doi.org/10.1016/j.nrl.2016.11.008.
Martinez AA, Morgese MG, Pisanu A, Macheda T, Paquette MA, Seillier A, et al. Activation of PPAR gamma receptors reduces levodopa-induced dyskinesias in 6-OHDA-lesioned rats. Neurobiol Dis. 2015;74:295–304. https://doi.org/10.1016/j.nbd.2014.11.024.
Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98(2):408–19. https://doi.org/10.1111/j.1471-4159.2006.03880.x.
Fernández-Suárez D, Celorrio M, Riezu-Boj JI, Ugarte A, Pacheco R, González H, et al. The monoacylglycerol lipase inhibitor JZL184 is neuroprotective and alters glial cell phenotype in the chronic MPTP mouse model. Neurobiol Aging. 2014;35(11):2603–16. https://doi.org/10.1016/j.neurobiolaging.2014.05.021.
van Vliet SAM, Vanwersch RAP, Jongsma MJ, Olivier B, Philippens IHCHM. Therapeutic effects of Δ9-THC and modafinil in a marmoset Parkinson model. Eur Neuropsychopharmacol. 2008;18(5):383–9. https://doi.org/10.1016/j.euroneuro.2007.11.003.
Fernandez-Espejo E, Caraballo I, de Fonseca FR, El Banoua F, Ferrer B, Flores JA, et al. Cannabinoid CB1 antagonists possess antiparkinsonian efficacy only in rats with very severe nigral lesion in experimental parkinsonism. Neurobiol Dis. 2005;18(3):591–601. https://doi.org/10.1016/j.nbd.2004.10.015.
Fernandez-Espejo E, Caraballo I, Rodriguez de Fonseca F, Ferrer B, Banoua FE, Flores JA, et al. Experimental parkinsonism alters anandamide precursor synthesis, and functional deficits are improved by AM404: a modulator of endocannabinoid function. Neuropsychopharmacology. 2004;29(6):1134–42. https://doi.org/10.1038/sj.npp.1300407.
González S, Scorticati C, García-Arencibia M, de Miguel R, Ramos JA, Fernández-Ruiz J. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson’s disease. Brain Res. 2006;1073–1074:209–19. https://doi.org/10.1016/j.brainres.2005.12.014.
Van Laere K, Casteels C, Lunskens S, Goffin K, Grachev ID, Bormans G, et al. Regional changes in type 1 cannabinoid receptor availability in Parkinson’s disease in vivo. Neurobiol Aging. 2012;33(3):620.e1-.e8. https://doi.org/10.1016/j.neurobiolaging.2011.02.009.
Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol Dis. 2005;19(1–2):96–107. https://doi.org/10.1016/j.nbd.2004.11.009.
Cerri S, Levandis G, Ambrosi G, Montepeloso E, Antoninetti GF, Franco R, et al. Neuroprotective potential of adenosine A2A and cannabinoid CB1 receptor antagonists in an animal model of Parkinson disease. J Neuropathol Exp Neurol. 2014;73(5):414–24. https://doi.org/10.1097/NEN.0000000000000064.
Price DA, Martinez AA, Seillier A, Koek W, Acosta Y, Fernandez E, et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29(11):2177–86. https://doi.org/10.1111/j.1460-9568.2009.06764.x.
Chung YC, Bok E, Huh SH, Park J-Y, Yoon S-H, Kim SR, et al. Cannabinoid receptor type 1 protects nigrostriatal dopaminergic neurons against MPTP neurotoxicity by inhibiting microglial activation. J Immunol. 2011;187(12):6508–17. https://doi.org/10.4049/jimmunol.1102435.
Lastres-Becker I, Cebeira M, de Ceballos ML, Zeng B-Y, Jenner P, Ramos JA, et al. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur J Neurosci. 2001;14(11):1827–32. https://doi.org/10.1046/j.0953-816x.2001.01812.x.
Martínez-Pinilla E, Aguinaga D, Navarro G, Rico AJ, Oyarzábal J, Sánchez-Arias JA, et al. Targeting CB(1) and GPR55 endocannabinoid receptors as a potential neuroprotective approach for Parkinson’s disease. Mol Neurobiol. 2019;56(8):5900–10. https://doi.org/10.1007/s12035-019-1495-4.
Zeissler M-L, Eastwood J, McCorry K, Hanemann CO, Zajicek JP, Carroll CB. Delta-9-tetrahydrocannabinol protects against MPP+ toxicity in SH-SY5Y cells by restoring proteins involved in mitochondrial biogenesis. Oncotarget. 2016;7(29):46603–14. https://doi.org/10.18632/oncotarget.10314.
Fishbein-Kaminietsky M, Gafni M, Sarne Y. Ultralow doses of cannabinoid drugs protect the mouse brain from inflammation-induced cognitive damage. J Neurosci Res. 2014;92(12):1669–77. https://doi.org/10.1002/jnr.23452.
McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172(3):737–53. https://doi.org/10.1111/bph.12944.
García C, Palomo-Garo C, García-Arencibia M, Ramos J, Pertwee R, Fernández-Ruiz J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson’s disease. Br J Pharmacol. 2011;163(7):1495–506. https://doi.org/10.1111/j.1476-5381.2011.01278.x.
Ojha S, Javed H, Azimullah S, Haque ME. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol Cell Biochem. 2016;418(1):59–70. https://doi.org/10.1007/s11010-016-2733-y.
Garcia C, Gomez-Canas M, Burgaz S, Palomares B, Gomez-Galvez Y, Palomo-Garo C, et al. Benefits of VCE-003.2, a cannabigerol quinone derivative, against inflammation-driven neuronal deterioration in experimental Parkinson’s disease: possible involvement of different binding sites at the PPARgamma receptor. J Neuroinflammation. 2018;15(1):19. https://doi.org/10.1186/s12974-018-1060-5.
Burgaz S, Garcia C, Gomez-Canas M, Munoz E, Fernandez-Ruiz J. Development of an oral treatment with the PPAR-gamma-acting cannabinoid VCE-003.2 against the inflammation-driven neuronal deterioration in experimental Parkinson’s disease. Molecules. 2019;24(15). https://doi.org/10.3390/molecules24152702.
Santos NAG, Martins NM, Sisti FM, Fernandes LS, Ferreira RS, Queiroz RHC, et al. The neuroprotection of cannabidiol against MPP+-induced toxicity in PC12 cells involves trkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson’s disease. Toxicol In Vitro. 2015;30(1, Part B):231–40. https://doi.org/10.1016/j.tiv.2015.11.004.
Martín-Moreno AM, Reigada D, Ramírez BG, Mechoulam R, Innamorato N, Cuadrado A, et al. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol Pharmacol. 2011;79(6):964–73. https://doi.org/10.1124/mol.111.071290.
Janefjord E, Mååg JLV, Harvey BS, Smid SD. Cannabinoid effects on β amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell Mol Neurobiol. 2014;34(1):31–42. https://doi.org/10.1007/s10571-013-9984-x.
Binda KH, Real CC, Ferreira AFF, Britto LR, Chacur M. Antinociceptive effects of treadmill exercise in a rat model of Parkinson’s disease: the role of cannabinoid and opioid receptors. Brain Res. 2019:146521. https://doi.org/10.1016/j.brainres.2019.146521.
Crivelaro do Nascimento G, Ferrari DP, Guimaraes FS, Del Bel EA, Bortolanza M, Ferreira-Junior NC. Cannabidiol increases the nociceptive threshold in a preclinical model of Parkinson’s disease. Neuropharmacology. 2020;163:107808. https://doi.org/10.1016/j.neuropharm.2019.107808.
Bisogno T, Hanuš L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845–52. https://doi.org/10.1038/sj.bjp.0704327.
Zuardi A, Crippa J, Hallak J, Pinto J, Chagas M, Rodrigues G, et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J Psychopharmacol. 2009;23(8):979–83. https://doi.org/10.1177/0269881108096519.
Chagas MHN, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28(11):1088–98. https://doi.org/10.1177/0269881114550355.
Chagas MHN, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther. 2014;39(5):564–6. https://doi.org/10.1111/jcpt.12179.
Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol. 2014;37(2):41–4. https://doi.org/10.1097/WNF.0000000000000016.
Kindred JH, Li K, Ketelhut NB, Proessl F, Fling BW, Honce JM, et al. Cannabis use in people with Parkinson’s disease and multiple sclerosis: a web-based investigation. Complement Ther Med. 2017;33:99–104. https://doi.org/10.1016/j.ctim.2017.07.002.
Peball M, Werkmann M, Ellmerer P, Stolz R, Valent D, Knaus H-G, et al. Nabilone for non-motor symptoms of Parkinson’s disease: a randomized placebo-controlled, double-blind, parallel-group, enriched enrolment randomized withdrawal study (the NMS-Nab Study). J Neural Transm (Vienna). 2019;126(8):1061–72. https://doi.org/10.1007/s00702-019-02021-z.
Pertwee RG. Cannabinoids and multiple sclerosis. Pharmacol Ther. 2002;95(2):165–74. https://doi.org/10.1016/s0163-7258(02)00255-3.
Compston A, Coles A. Multiple sclerosis. Lancet (London, England). 2008;372(9648):1502–17. https://doi.org/10.1016/s0140-6736(08)61620-7.
Nielsen S, Germanos R, Weier M, Pollard J, Degenhardt L, Hall W, et al. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of reviews. Curr Neurol Neurosci Rep. 2018;18(2):8. https://doi.org/10.1007/s11910-018-0814-x.
Gaby A. Multiple sclerosis. Global Adv Health Med. 2013;2(1):50–6. https://doi.org/10.7453/gahmj.2013.2.1.009.
Nicholas R, Rashid W. Multiple sclerosis. BMJ Clin Evid. 2012;2012:1202.
Nicholas R, Rashid W. Multiple sclerosis. Am Fam Physician. 2013;87(10):712–4.
Uzunkopru C, Beckmann Y. Flammer syndrome in multiple sclerosis: diagnostics, prediction, and personalization of treatments. EPMA J. 2019;10(4):437–44. https://doi.org/10.1007/s13167-019-00179-w.
Goncharenko V, Bubnov R, Polivka J Jr, Zubor P, Biringer K, Bielik T, et al. Vaginal dryness: individualised patient profiles, risks and mitigating measures. EPMA J. 2019;10(1):73–9. https://doi.org/10.1007/s13167-019-00164-3.
Golubnitschaja O, Flammer J. Individualised patient profile: clinical utility of Flammer syndrome phenotype and general lessons for predictive, preventive and personalised medicine. EPMA J. 2018;9(1):15–20. https://doi.org/10.1007/s13167-018-0127-9.
Cabranes A, Venderova K, de Lago E, Fezza F, Sanchez A, Mestre L, et al. Decreased endocannabinoid levels in the brain and beneficial effects of agents activating cannabinoid and/or vanilloid receptors in a rat model of multiple sclerosis. Neurobiol Dis. 2005;20(2):207–17. https://doi.org/10.1016/j.nbd.2005.03.002.
Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15(2):300–2. https://doi.org/10.1096/fj.00-0399fje.
Chiurchiù V, van der Stelt M, Centonze D, Maccarrone M. The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: clues for other neuroinflammatory diseases. Prog Neurobiol. 2018;160:82–100. https://doi.org/10.1016/j.pneurobio.2017.10.007.
Di Filippo M, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P. Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79(11):1224–9. https://doi.org/10.1136/jnnp.2007.139071.
Petro DJ, Ellenberger C Jr. Treatment of human spasticity with delta 9-tetrahydrocannabinol. J Clin Pharmacol. 1981;21(S1):413s–6s. https://doi.org/10.1002/j.1552-4604.1981.tb02621.x.
Ungerleider JT, Andrysiak T. Therapeutic issues of marijuana and THC (tetrahydrocannabinol). Int J Addict. 1985;20(5):691–9. https://doi.org/10.3109/10826088509044289.
Meinck HM, Schonle PW, Conrad B. Effect of cannabinoids on spasticity and ataxia in multiple sclerosis. J Neurol. 1989;236(2):120–2. https://doi.org/10.1007/bf00314410.
Clifford DB. Tetrahydrocannabinol for tremor in multiple sclerosis. Ann Neurol. 1983;13(6):669–71. https://doi.org/10.1002/ana.410130616.
Pryce G, Riddall DR, Selwood DL, Giovannoni G, Baker D. Neuroprotection in experimental autoimmune encephalomyelitis and progressive multiple sclerosis by cannabis-based cannabinoids. J Neuroimmune Pharmacol. 2015;10(2):281–92. https://doi.org/10.1007/s11481-014-9575-8.
Colizzi M, Bhattacharyya S. Does cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Curr Addict Rep. 2017;4(2):62–74. https://doi.org/10.1007/s40429-017-0142-2.
Rudroff T. Cannabis for neuropathic pain in multiple sclerosis-high expectations. Poor Data Front Pharmacol. 2019;10:1239. https://doi.org/10.3389/fphar.2019.01239.
Rudroff T, Honce JM. Cannabis and multiple sclerosis-the way forward. Front Neurol. 2017;8:299. https://doi.org/10.3389/fneur.2017.00299.
Wilkinson JD, Whalley BJ, Baker D, Pryce G, Constanti A, Gibbons S, et al. Medicinal cannabis: is delta9-tetrahydrocannabinol necessary for all its effects? J Pharm Pharmacol. 2003;55(12):1687–94. https://doi.org/10.1211/0022357022304.
Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol. 2007;150(4):519–25. https://doi.org/10.1038/sj.bjp.0707003.
Perez J. Combined cannabinoid therapy via an oromucosal spray. Drugs Today (Barcelona, Spain : 1998). 2006;42(8):495–503. https://doi.org/10.1358/dot.2006.42.8.1021517.
Al-Ghezi ZZ, Miranda K, Nagarkatti M, Nagarkatti PS. Combination of cannabinoids, delta9-tetrahydrocannabinol and cannabidiol ameliorates experimental multiple sclerosis by suppressing neuroinflammation through regulation of miRNA-mediated signaling pathways. Front Immunol. 2019;10:1921. https://doi.org/10.3389/fimmu.2019.01921.
Meuth SG, Vila C, Dechant KL. Effect of Sativex on spasticity-associated symptoms in patients with multiple sclerosis. Expert Rev Neurother. 2015;15(8):909–18. https://doi.org/10.1586/14737175.2015.1067607.
Gado F, Digiacomo M, Macchia M, Bertini S, Manera C. Traditional uses of cannabinoids and new perspectives in the treatment of multiple sclerosis. Medicines (Basel, Switzerland). 2018;5(3). https://doi.org/10.3390/medicines5030091.
Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ (Clinical Research Ed). 2004;329(7460):253. https://doi.org/10.1136/bmj.38149.566979.AE.
Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet (London, England). 2003;362(9395):1517–26. https://doi.org/10.1016/s0140-6736(03)14738-1.
Zajicek JP, Apostu VI. Role of cannabinoids in multiple sclerosis. CNS Drugs. 2011;25(3):187–201. https://doi.org/10.2165/11539000-000000000-00000.
Wissel J, Haydn T, Muller J, Brenneis C, Berger T, Poewe W, et al. Low dose treatment with the synthetic cannabinoid nabilone significantly reduces spasticity-related pain : a double-blind placebo-controlled cross-over trial. J Neurol. 2006;253(10):1337–41. https://doi.org/10.1007/s00415-006-0218-8.
Gehr S, Kaiser T, Kreutz R, Ludwig WD, Paul F. Suggestions for improving the design of clinical trials in multiple sclerosis-results of a systematic analysis of completed phase III trials. EPMA J. 2019;10(4):425–36. https://doi.org/10.1007/s13167-019-00192-z.
Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23(35):11136–41.
Piro JR, Benjamin DI, Duerr JM, Pi Y, Gonzales C, Wood KM, et al. A dysregulated endocannabinoid-eicosanoid network supports pathogenesis in a mouse model of Alzheimer’s disease. Cell Rep. 2012;1(6):617–23. https://doi.org/10.1016/j.celrep.2012.05.001.
Pazos MR, Nunez E, Benito C, Tolon RM, Romero J. Role of the endocannabinoid system in Alzheimer’s disease: new perspectives. Life Sci. 2004;75(16):1907–15. https://doi.org/10.1016/j.lfs.2004.03.026.
Mulder J, Zilberter M, Pasquare SJ, Alpar A, Schulte G, Ferreira SG, et al. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain. 2011;134(Pt 4):1041–60. https://doi.org/10.1093/brain/awr046.
Koppel J, Davies P. Targeting the endocannabinoid system in Alzheimer’s disease. J Alzheimers Dis. 2008;15(3):495–504.
Karl T, Cheng D, Garner B, Arnold JC. The therapeutic potential of the endocannabinoid system for Alzheimer’s disease. Expert Opin Ther Targets. 2012;16(4):407–20. https://doi.org/10.1517/14728222.2012.671812.
Fernandez-Ruiz J, Romero J, Ramos JA. Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and others. Handb Exp Pharmacol. 2015;231:233–59. https://doi.org/10.1007/978-3-319-20825-1_8.
Campbell VA, Gowran A. Alzheimer’s disease; taking the edge off with cannabinoids? Br J Pharmacol. 2007;152(5):655–62. https://doi.org/10.1038/sj.bjp.0707446.
Benito C, Nunez E, Pazos MR, Tolon RM, Romero J. The endocannabinoid system and Alzheimer’s disease. Mol Neurobiol. 2007;36(1):75–81. https://doi.org/10.1007/s12035-007-8006-8.
Bedse G, Romano A, Lavecchia AM, Cassano T, Gaetani S. The role of endocannabinoid signaling in the molecular mechanisms of neurodegeneration in Alzheimer’s disease. J Alzheimers Dis. 2015;43(4):1115–36. https://doi.org/10.3233/JAD-141635.
Aso E, Ferrer I. CB2 cannabinoid receptor as potential target against Alzheimer’s disease. Front Neurosci. 2016;10:243. https://doi.org/10.3389/fnins.2016.00243.
Talarico G, Trebbastoni A, Bruno G, de Lena C. Modulation of the cannabinoid system: a new perspective for the treatment of the Alzheimer’s disease. Curr Neuropharmacol. 2019;17(2):176–83. https://doi.org/10.2174/1570159X16666180702144644.
Ahmad R, Postnov A, Bormans G, Versijpt J, Vandenbulcke M, Van Laere K. Decreased in vivo availability of the cannabinoid type 2 receptor in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2016;43(12):2219–27. https://doi.org/10.1007/s00259-016-3457-7.
Rodríguez-Cueto C, Benito C, Fernández-Ruiz J, Romero J, Hernández-Gálvez M, Gómez-Ruiz M. Changes in CB(1) and CB(2) receptors in the post-mortem cerebellum of humans affected by spinocerebellar ataxias. Br J Pharmacol. 2014;171(6):1472–89. https://doi.org/10.1111/bph.12283.
Manuel I, González de San Román E, Giralt MT, Ferrer I, Rodríguez-Puertas R. Type-1 cannabinoid receptor activity during Alzheimer’s disease progression. J Alzheimers Dis. 2014;42(3):761–6. https://doi.org/10.3233/JAD-140492.
Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 2015;16(1):30–42. https://doi.org/10.1038/nrn3876.
Takkinen JS, López-Picón FR, Kirjavainen AK, Pihlaja R, Snellman A, Ishizu T, et al. [(18)F]FMPEP-d(2) PET imaging shows age- and genotype-dependent impairments in the availability of cannabinoid receptor 1 in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2018;69:199–208. https://doi.org/10.1016/j.neurobiolaging.2018.05.013.
Ahmad R, Goffin K, Van den Stock J, De Winter F-L, Cleeren E, Bormans G, et al. In vivo type 1 cannabinoid receptor availability in Alzheimer’s disease. Eur Neuropsychopharmacol. 2014;24(2):242–50. https://doi.org/10.1016/j.euroneuro.2013.10.002.
Maccarrone M, Totaro A, Leuti A, Giacovazzo G, Scipioni L, Mango D, et al. Early alteration of distribution and activity of hippocampal type-1 cannabinoid receptor in Alzheimer’s disease-like mice overexpressing the human mutant amyloid precursor protein. Pharmacol Res. 2018;130:366–73. https://doi.org/10.1016/j.phrs.2018.02.009.
Köfalvi A, Lemos C, Martín-Moreno AM, Pinheiro BS, García-García L, Pozo MA, et al. Stimulation of brain glucose uptake by cannabinoid CB2 receptors and its therapeutic potential in Alzheimer’s disease. Neuropharmacology. 2016;110(Pt A):519–29. https://doi.org/10.1016/j.neuropharm.2016.03.015.
Pascual AC, Martín-Moreno AM, Giusto NM, de Ceballos ML, Pasquaré SJ. Normal aging in rats and pathological aging in human Alzheimer’s disease decrease FAAH activity: modulation by cannabinoid agonists. Exp Gerontol. 2014;60:92–9. https://doi.org/10.1016/j.exger.2014.10.011.
Zhang J, Chen C. Alleviation of neuropathology by inhibition of monoacylglycerol lipase in APP transgenic mice lacking CB2 receptors. Mol Neurobiol. 2018;55(6):4802–10. https://doi.org/10.1007/s12035-017-0689-x.
Cassano T, Calcagnini S, Pace L, De Marco F, Romano A, Gaetani S. Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci. 2017;11:–30. https://doi.org/10.3389/fnins.2017.00030.
Jayant S, Sharma BM, Bansal R, Sharma B. Pharmacological benefits of selective modulation of cannabinoid receptor type 2 (CB2) in experimental Alzheimer’s disease. Pharmacol Biochem Behav. 2016;140:39–50. https://doi.org/10.1016/j.pbb.2015.11.006.
Cheng D, Low JK, Logge W, Garner B, Karl T. Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1∆E9 mice. Psychopharmacology. 2014;231(15):3009–17. https://doi.org/10.1007/s00213-014-3478-5.
Vallée A, Lecarpentier Y, Guillevin R, Vallée J-N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim Biophys Sin Shanghai. 2017;49(10):853–66. https://doi.org/10.1093/abbs/gmx073.
Karl T, Garner B, Cheng D. The therapeutic potential of the phytocannabinoid cannabidiol for Alzheimer’s disease. Behav Pharmacol. 2017;28(2 and 3-Spec Issue):142–60. https://doi.org/10.1097/FBP.0000000000000247.
Wang L, Liu B-J, Cao Y, Xu W-Q, Sun D-S, Li M-Z, et al. Deletion of type-2 cannabinoid receptor induces Alzheimer’s disease-like tau pathology and memory impairment through AMPK/GSK3β pathway. Mol Neurobiol. 2018;55(6):4731–44. https://doi.org/10.1007/s12035-017-0676-2.
Aso E, Andrés-Benito P, Carmona M, Maldonado R, Ferrer I. Cannabinoid receptor 2 participates in amyloid-β processing in a mouse model of Alzheimer’s disease but plays a minor role in the therapeutic properties of a cannabis-based medicine. J Alzheimers Dis. 2016;51(2):489–500. https://doi.org/10.3233/JAD-150913.
Schmöle A-C, Lundt R, Toporowski G, Hansen JN, Beins E, Halle A, et al. Cannabinoid receptor 2-deficiency ameliorates disease symptoms in a mouse model with Alzheimer’s disease-like pathology. J Alzheimers Dis. 2018;64(2):379–92. https://doi.org/10.3233/JAD-180230.
Zanettini C, Panlilio L, Aliczki M, Goldberg S, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5(57). https://doi.org/10.3389/fnbeh.2011.00057.
Nadia S, Robert B. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1(1):81–98. https://doi.org/10.2174/1874473710801010081.
Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–43. https://doi.org/10.1212/01.wnl.0000031422.66442.49.
Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81(2):319–30. https://doi.org/10.1016/j.pbb.2005.02.017.
Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, et al. Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry. 1996;53(11):1051–7. https://doi.org/10.1001/archpsyc.1996.01830110089011.
Iversen L, Chapman V. Cannabinoids: a real prospect for pain relief? Curr Opin Pharmacol. 2002;2(1):50–5.
Calabrese EJ, Rubio-Casillas A. Biphasic effects of THC in memory and cognition. Eur J Clin Investig. 2018;48(5):e12920-e. https://doi.org/10.1111/eci.12920.
Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji S-P, Bai G, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115(11):3104–16. https://doi.org/10.1172/JCI25509.
Assaf F, Fishbein M, Gafni M, Keren O, Sarne Y. Pre- and post-conditioning treatment with an ultra-low dose of Δ9-tetrahydrocannabinol (THC) protects against pentylenetetrazole (PTZ)-induced cognitive damage. Behav Brain Res. 2011;220(1):194–201. https://doi.org/10.1016/j.bbr.2011.02.005.
Bilkei-Gorzo A, Albayram O, Draffehn A, Michel K, Piyanova A, Oppenheimer H, et al. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med. 2017;23(6):782–7. https://doi.org/10.1038/nm.4311.
Sarne Y, Toledano R, Rachmany L, Sasson E, Doron R. Reversal of age-related cognitive impairments in mice by an extremely low dose of tetrahydrocannabinol. Neurobiol Aging. 2018;61:177–86. https://doi.org/10.1016/j.neurobiolaging.2017.09.025.
Shelef A, Barak Y, Berger U, Paleacu D, Tadger S, Plopsky I, et al. Safety and efficacy of medical cannabis oil for behavioral and psychological symptoms of dementia: an-open label, add-on, pilot study. J Alzheimers Dis. 2016;51(1):15–9. https://doi.org/10.3233/JAD-150915.
van den Elsen GAH, Ahmed AIA, Lammers M, Kramers C, Verkes RJ, van der Marck MA, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014;14:56–64. https://doi.org/10.1016/j.arr.2014.01.007.
Liu CS, Chau SA, Ruthirakuhan M, Lanctôt KL, Herrmann N. Cannabinoids for the treatment of agitation and aggression in Alzheimer’s disease. CNS Drugs. 2015;29(8):615–23. https://doi.org/10.1007/s40263-015-0270-y.
Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Santamato A, Lozupone M, et al. Progresses in treating agitation: a major clinical challenge in Alzheimer’s disease. Expert Opin Pharmacother. 2015;16(17):2581–8. https://doi.org/10.1517/14656566.2015.1092520.
Porsteinsson AP, Antonsdottir IM. An update on the advancements in the treatment of agitation in Alzheimer’s disease. Expert Opin Pharmacother. 2017;18(6):611–20. https://doi.org/10.1080/14656566.2017.1307340.
Ruthirakuhan M, Lanctôt KL, Vieira D, Herrmann N. Natural and synthetic cannabinoids for agitation and aggression in Alzheimer’s disease: a meta-analysis. J Clin Psychiatry. 2019;80(2):18r12617. https://doi.org/10.4088/JCP.18r12617.
Ruthirakuhan MT, Herrmann N, Gallagher D, Andreazza AC, Kiss A, Verhoeff NPLG, et al. Investigating the safety and efficacy of nabilone for the treatment of agitation in patients with moderate-to-severe Alzheimer’s disease: study protocol for a cross-over randomized controlled trial. Contemp Clin Trials Commun. 2019;15:100385. https://doi.org/10.1016/j.conctc.2019.100385.
Zamberletti E, Rubino T, Parolaro D. The endocannabinoid system and schizophrenia: integration of evidence. Curr Pharm Des. 2012;18(32):4980–90. https://doi.org/10.2174/138161212802884744.
Vigano D, Guidali C, Petrosino S, Realini N, Rubino T, Di Marzo V, et al. Involvement of the endocannabinoid system in phencyclidine-induced cognitive deficits modelling schizophrenia. Int J Neuropsychopharmacol. 2009;12(5):599–614. https://doi.org/10.1017/S1461145708009371.
Ujike H, Morita Y. New perspectives in the studies on endocannabinoid and cannabis: cannabinoid receptors and schizophrenia. J Pharmacol Sci. 2004;96(4):376–81. https://doi.org/10.1254/jphs.fmj04003x4.
Schwarcz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255–8. https://doi.org/10.1097/JCP.0b013e3181a6bc3b.
Muguruza C, Lehtonen M, Aaltonen N, Morentin B, Meana JJ, Callado LF. Quantification of endocannabinoids in postmortem brain of schizophrenic subjects. Schizophr Res. 2013;148(1–3):145–50. https://doi.org/10.1016/j.schres.2013.06.013.
Koethe D, Llenos IC, Dulay JR, Hoyer C, Torrey EF, Leweke FM, et al. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm (Vienna). 2007;114(8):1055–63. https://doi.org/10.1007/s00702-007-0660-5.
Fernandez-Espejo E, Viveros MP, Nunez L, Ellenbroek BA, Rodriguez de Fonseca F. Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology. 2009;206(4):531–49. https://doi.org/10.1007/s00213-009-1612-6.
De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. https://doi.org/10.1186/1476-511X-2-5.
Cohen M, Solowij N, Carr V. Cannabis, cannabinoids and schizophrenia: integration of the evidence. Aust N Z J Psychiatry. 2008;42(5):357–68. https://doi.org/10.1080/00048670801961156.
Chavarría-Siles I, Contreras-Rojas J, Hare E, Walss-Bass C, Quezada P, Dassori A, et al. Cannabinoid receptor 1 gene (CNR1) and susceptibility to a quantitative phenotype for hebephrenic schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147(3):279–84. https://doi.org/10.1002/ajmg.b.30592.
Martínez-Gras I, Hoenicka J, Ponce G, Rodríguez–Jiménez R, Jiménez-Arriero MA, Pérez-Hernandez E et al. (AAT)n repeat in the cannabinoid receptor gene, CNR1: association with schizophrenia in a Spanish population. Eur Arch Psychiatry Clin Neurosci 2006;256(7):437–441. doi:https://doi.org/10.1007/s00406-006-0665-3.
Suárez-Pinilla P, Roiz-Santiañez R, Ortiz-García de la Foz V, Guest PC, Ayesa-Arriola R, Córdova-Palomera A, et al. Brain structural and clinical changes after first episode psychosis: focus on cannabinoid receptor 1 polymorphisms. Psychiatry Res Neuroimaging 2015;233(2):112–119. doi:https://doi.org/10.1016/j.pscychresns.2015.05.005.
Ujike H, Takaki M, Nakata K, Tanaka Y, Takeda T, Kodama M, et al. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol Psychiatry. 2002;7(5):515–8. https://doi.org/10.1038/sj.mp.4001029.
Ho B-C, Wassink TH, Ziebell S, Andreasen NC. Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr Res. 2011;128(1):66–75. https://doi.org/10.1016/j.schres.2011.02.021.
Ishiguro H, Horiuchi Y, Ishikawa M, Koga M, Imai K, Suzuki Y, et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry. 2010;67(10):974–82. https://doi.org/10.1016/j.biopsych.2009.09.024.
Muller-Vahl KR. Treatment of Tourette syndrome with cannabinoids. Behav Neurol. 2013;27(1):119–24. https://doi.org/10.3233/BEN-120276.
Ceccarini J, De Hert M, van Winkel R, Koethe D, Bormans G, Leweke M, et al. In vivo pet imaging of cerebral type 1 cannabinoid receptor availability in patients with schizophrenia. Schizophr Res. 2010;117(2):170. https://doi.org/10.1016/j.schres.2010.02.196.
Dalton VS, Long LE, Weickert CS, Zavitsanou K. Paranoid schizophrenia is characterized by increased CB1 receptor binding in the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2011;36(8):1620–30. https://doi.org/10.1038/npp.2011.43.
Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103(1):9–15. https://doi.org/10.1016/S0306-4522(00)00552-2.
Eggan SM, Stoyak SR, Verrico CD, Lewis DA. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology. 2010;35(10):2060–71. https://doi.org/10.1038/npp.2010.75.
Urigüen L, García-Fuster MJ, Callado LF, Morentin B, La Harpe R, Casadó V, et al. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology. 2009;206(2):313–24. https://doi.org/10.1007/s00213-009-1608-2.
Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. NeuroImage. 2010;52(4):1505–13. https://doi.org/10.1016/j.neuroimage.2010.04.034.
Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28(2):355–60. https://doi.org/10.1016/j.pnpbp.2003.11.005.
Reuter AR, Bumb JM, Mueller JK, Rohleder C, Pahlisch F, Hanke F, et al. Association of anandamide with altered binocular depth inversion illusion in schizophrenia. World J Biol Psychiatry. 2017;18(6):483–8. https://doi.org/10.1080/15622975.2016.1246750.
Pushpa-Rajah JA, McLoughlin BC, Gillies D, Rathbone J, Variend H, Kalakouti E, et al. Cannabis and schizophrenia. Schizophr Bull. 2015;41(2):336–7. https://doi.org/10.1093/schbul/sbu168.
Potvin S, Stip E, Lipp O, Roy M-A, Demers M-F, Bouchard R-H, et al. Anhedonia and social adaptation predict substance abuse evolution in dual diagnosis schizophrenia. Am J Drug Alcohol Abuse. 2008;34(1):75–82. https://doi.org/10.1080/00952990701764631.
Almeida V, Peres FF, Levin R, Suiama MA, Calzavara MB, Zuardi AW, et al. Effects of cannabinoid and vanilloid drugs on positive and negative-like symptoms on an animal model of schizophrenia: the SHR strain. Schizophr Res. 2014;153(1):150–9. https://doi.org/10.1016/j.schres.2014.01.039.
Levin R, Peres FF, Almeida V, Calzavara MB, Zuardi AW, Hallak JEC, et al. Effects of cannabinoid drugs on the deficit of prepulse inhibition of startle in an animal model of schizophrenia: the SHR strain. Front Pharmacol. 2014;5:10. https://doi.org/10.3389/fphar.2014.00010.
Malone DT, Taylor DA. The effect of Δ9-tetrahydrocannabinol on sensorimotor gating in socially isolated rats. Behav Brain Res. 2006;166(1):101–9. https://doi.org/10.1016/j.bbr.2005.07.009.
Schneider M, Koch M. The cannabinoid agonist WIN 55,212-2 reduces sensorimotor gating and recognition memory in rats. Behav Pharmacol. 2002;13(1):29–37. https://doi.org/10.1097/00008877-200202000-00003.
Wegener N, Kuhnert S, Thüns A, Roese R, Koch M. Effects of acute systemic and intra-cerebral stimulation of cannabinoid receptors on sensorimotor gating, locomotion and spatial memory in rats. Psychopharmacology. 2008;198(3):375. https://doi.org/10.1007/s00213-008-1148-1.
Ballmaier M, Bortolato M, Rizzetti C, Zoli M, Gessa G, Heinz A, et al. Cannabinoid receptor antagonists counteract sensorimotor gating deficits in the phencyclidine model of psychosis. Neuropsychopharmacology. 2007;32(10):2098–107. https://doi.org/10.1038/sj.npp.1301344.
Raquel L, Valeria A, Fernanda Fiel P, Mariana Bendlin C, Neide Derci da S, Mayra Akimi S, et al. Antipsychotic profile of cannabidiol and rimonabant in an animal model of emotional context processing in schizophrenia. Curr Pharm Des. 2012;18(32):4960–5. https://doi.org/10.2174/138161212802884735.
Campos AC, Rocha NP, Nicoli JR, Vieira LQ, Teixeira MM, Teixeira AL. Absence of gut microbiota influences lipopolysaccharide-induced behavioral changes in mice. Behav Brain Res. 2016;312:186–94. https://doi.org/10.1016/j.bbr.2016.06.027.
Peres FF, Diana MC, Suiama MA, Justi V, Almeida V, Bressan RA, et al. Peripubertal treatment with cannabidiol prevents the emergence of psychosis in an animal model of schizophrenia. Schizophr Res. 2016;172(1):220–1. https://doi.org/10.1016/j.schres.2016.02.004.
Zuardi AW, Morais SL, Guimarães FS, Mechoulam R. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485–6.
Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94-e. https://doi.org/10.1038/tp.2012.15.
Philip McGuire FRC, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatr. 2018;175(3):225–31. https://doi.org/10.1176/appi.ajp.2017.17030325.
Amare AT, Schubert KO, Baune BT. Pharmacogenomics in the treatment of mood disorders: strategies and opportunities for personalized psychiatry. EPMA J. 2017;8(3):211–27. https://doi.org/10.1007/s13167-017-0112-8.
Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol. 2004;68(9):1691–8. https://doi.org/10.1016/j.bcp.2004.07.007.
Szaflarski JP, Bebin EM. Cannabis, cannabidiol, and epilepsy—from receptors to clinical response. Epilepsy Behav. 2014;41:277–82. https://doi.org/10.1016/j.yebeh.2014.08.135.
Ludanyi A, Eross L, Czirjak S, Vajda J, Halasz P, Watanabe M, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28(12):2976–90. https://doi.org/10.1523/JNEUROSCI.4465-07.2008.
Goffin K, Van Paesschen W, Van Laere K. In vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain. 2011;134(Pt 4):1033–40. https://doi.org/10.1093/brain/awq385.
von Rüden EL, Bogdanovic RM, Wotjak CT, Potschka H. Inhibition of monoacylglycerol lipase mediates a cannabinoid 1-receptor dependent delay of kindling progression in mice. Neurobiol Dis. 2015;77:238–45. https://doi.org/10.1016/j.nbd.2015.03.016.
Terrone G, Pauletti A, Salamone A, Rizzi M, Villa BR, Porcu L, et al. Inhibition of monoacylglycerol lipase terminates diazepam-resistant status epilepticus in mice and its effects are potentiated by a ketogenic diet. Epilepsia. 2018;59(1):79–91. https://doi.org/10.1111/epi.13950.
Naziroglu M, Taner AN, Balbay E, Cig B. Inhibitions of anandamide transport and FAAH synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1 channel in an in vitro seizure model. Mol Cell Biochem. 2018. https://doi.org/10.1007/s11010-018-3439-0.
Colangeli R, Pierucci M, Benigno A, Campiani G, Butini S, Di Giovanni G. The FAAH inhibitor URB597 suppresses hippocampal maximal dentate afterdischarges and restores seizure-induced impairment of short and long-term synaptic plasticity. Sci Rep. 2017;7(1):11152. https://doi.org/10.1038/s41598-017-11606-1.
Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12(2):195–204.
Woodhams SG, Sagar DR, Burston JJ, Chapman V. The role of the endocannabinoid system in pain. Handb Exp Pharmacol. 2015;227:119–43. https://doi.org/10.1007/978-3-662-46450-2_7.
Mallet C, Daulhac L, Bonnefont J, Ledent C, Etienne M, Chapuy E, et al. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139(1):190–200. https://doi.org/10.1016/j.pain.2008.03.030.
Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, et al. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330(3):902–10. https://doi.org/10.1124/jpet.109.155465.
Guindon J, Beaulieu P. The role of the endogenous cannabinoid system in peripheral analgesia. Curr Mol Pharmacol. 2009;2(1):134–9.
Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13(10):1265–70. https://doi.org/10.1038/nn.2632.
Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther. 2004;308(2):446–53. https://doi.org/10.1124/jpet.103.060079.
Malan TP Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93(3):239–45. https://doi.org/10.1016/s0304-3959(01)00321-9.
Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529–33. https://doi.org/10.1073/pnas.1834309100.
Calignano A, La Rana G, Piomelli D. Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur J Pharmacol. 2001;419(2–3):191–8. https://doi.org/10.1016/s0014-2999(01)00988-8.
Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63(5):569–611.
Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids. 2002;121(1–2):173–90.
Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol. 2005;168:509–54.
Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol. 2007;152(5):765–77. https://doi.org/10.1038/sj.bjp.0707333.
Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23(6):1530–8. https://doi.org/10.1111/j.1460-9568.2006.04684.x.
Khan SP, Pickens TA, Berlau DJ. Perspectives on cannabis as a substitute for opioid analgesics. Pain Manag. 2019;9(2):191–203. https://doi.org/10.2217/pmt-2018-0051.
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556–63. https://doi.org/10.1212/wnl.0000000000000363.
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–73. https://doi.org/10.1001/jama.2015.6358.
Kim PS, Fishman MA. Cannabis for pain and headaches: primer. Curr Pain Headache Rep. 2017;21(4):19. https://doi.org/10.1007/s11916-017-0619-7.
NIDA. Marijuana. 2019. https://www.drugabuse.gov/publications/research-reports/marijuana. Accessed 22 November 2019.
NIDA. Marijuana as medicine. National Institute of Drug Abuse. 2019. https://www.drugabuse.gov/publications/drugfacts/marijuana-medicine. Accessed 22 Novemeber, 2019 2019.
Grant I. Medical use of cannabinoids. JAMA. 2015;314(16):1750–1. https://doi.org/10.1001/jama.2015.11429.
Weizman L, Dayan L, Brill S, Nahman-Averbuch H, Hendler T, Jacob G, et al. Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology. 2018;91(14):e1285–e94. https://doi.org/10.1212/wnl.0000000000006293.
de Robles P, Fiest KM, Frolkis AD, Pringsheim T, Atta C, St Germaine-Smith C, et al. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro Oncol. 2015;17(6):776–83. https://doi.org/10.1093/neuonc/nou283.
Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18(1):3–9. https://doi.org/10.22034/apjcp.2017.18.1.3.
Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–20. https://doi.org/10.1038/s41571-019-0177-5.
Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18(4):376–93. doi:https://doi.org/10.1016/s1474-4422(18)30468-x.
Anjum K, Shagufta BI, Abbas SQ, Patel S, Khan I, Shah SAA, et al. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: a review. Biomed Pharmacother. 2017;92:681–9. https://doi.org/10.1016/j.biopha.2017.05.125.
Bifulco M, Di Marzo V. Targeting the endocannabinoid system in cancer therapy: a call for further research. Nat Med. 2002;8(6):547–50. https://doi.org/10.1038/nm0602-547.
Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–80.
Di Marzo V. ‘Endocannabinoids’ and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochim Biophys Acta. 1998;1392(2–3):153–75.
Munson A, Harris L, Friedman M, Dewey W, Carchman RA. Antineoplastic activity of cannabinoids. J Natl Cancer Inst. 1975;55(3):597–602.
Guzmán M, Sánchez C, Galve-Roper. Control of the cell survival/death decision by cannabinoids. J Mol Med (Berl). 2001;78(11):613–25.
Massi P, Vaccani A, Ceruti S, Colombo A, Abbracchio MP, Parolaro D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther. 2004;308(3):838–45. https://doi.org/10.1124/jpet.103.061002.
Singer E, Judkins J, Salomonis N, Matlaf L, Soteropoulos P, McAllister S, et al. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. https://doi.org/10.1038/cddis.2014.566.
Hermanson DJ, Marnett LJ. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011;30(3–4):599–612. https://doi.org/10.1007/s10555-011-9318-8.
Galanti G, Fisher T, Kventsel I, Shoham J, Gallily R, Mechoulam R, et al. Delta 9-tetrahydrocannabinol inhibits cell cycle progression by downregulation of E2F1 in human glioblastoma multiforme cells. Acta Oncologica (Stockholm, Sweden). 2008;47(6):1062–70. https://doi.org/10.1080/02841860701678787.
Hernandez-Tiedra S, Fabrias G, Davila D, Salanueva IJ, Casas J, Montes LR, et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy. 2016;12(11):2213–29. https://doi.org/10.1080/15548627.2016.1213927.
Galve-Roperh I, Sanchez C, Cortes ML, Gomez del Pulgar T, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6(3):313–9. https://doi.org/10.1038/73171.
Lopez-Valero I, Torres S, Salazar-Roa M, Garcia-Taboada E, Hernandez-Tiedra S, Guzman M, et al. Optimization of a preclinical therapy of cannabinoids in combination with temozolomide against glioma. Biochem Pharmacol. 2018;157:275–84. https://doi.org/10.1016/j.bcp.2018.08.023.
Ellert-Miklaszewska A, Ciechomska I, Kaminska B. Cannabinoid signaling in glioma cells. Adv Exp Med Biol. 2013;986:209–20. https://doi.org/10.1007/978-94-007-4719-7_11.
Ellert-Miklaszewska A, Grajkowska W, Gabrusiewicz K, Kaminska B, Konarska L. Distinctive pattern of cannabinoid receptor type II (CB2) expression in adult and pediatric brain tumors. Brain Res. 2007;1137(1):161–9. https://doi.org/10.1016/j.brainres.2006.12.060.
Cioni C, Tassi M, Marotta G, Mugnaini C, Corelli F, Annunziata P. A novel highly selective cannabinoid CB2 agonist reduces in vitro growth and TGF-beta release of human glial cell tumors. Cent Nerv Syst Agents Med Chem. 2019;19(3):206–14. https://doi.org/10.2174/1871524919666190923154351.
Blazquez C, Casanova ML, Planas A, Gomez Del Pulgar T, Villanueva C, Fernandez-Acenero MJ, et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003;17(3):529–31. https://doi.org/10.1096/fj.02-0795fje.
Nabissi M, Morelli MB, Santoni M, Santoni G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2013;34(1):48–57. https://doi.org/10.1093/carcin/bgs328.
Nabissi M, Morelli MB, Amantini C, Farfariello V, Ricci-Vitiani L, Caprodossi S, et al. TRPV2 channel negatively controls glioma cell proliferation and resistance to Fas-induced apoptosis in ERK-dependent manner. Carcinogenesis. 2010;31(5):794–803. https://doi.org/10.1093/carcin/bgq019.
Likar R, Nahler G. The use of cannabis in supportive care and treatment of brain tumor. Neurooncol Pract. 2017;4(3):151–60. https://doi.org/10.1093/nop/npw027.
Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. 2019;24(4):549–54. https://doi.org/10.1634/theoncologist.2018-0383.
Likar R, Koestenberger M, Stultschnig M, Nahler G. Concomitant treatment of malignant brain tumours with CBD—a case series and review of the literature. Anticancer Res. 2019;39(10):5797–801. https://doi.org/10.21873/anticanres.13783.
Warren PP, Bebin EM, Nabors LB, Szaflarski JP. The use of cannabidiol for seizure management in patients with brain tumor-related epilepsy. Neurocase. 2017;23(5–6):287–91. https://doi.org/10.1080/13554794.2017.1391294.
Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16(1):9–29. https://doi.org/10.1038/s41582-019-0284-z.
Dumitru CA, Sandalcioglu IE, Karsak M. Cannabinoids in glioblastoma therapy: new applications for old drugs. Front Mol Neurosci. 2018;11:159. https://doi.org/10.3389/fnmol.2018.00159.
Janssens JP, Schuster K, Voss A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018;9(2):113–23. https://doi.org/10.1007/s13167-018-0130-1.
Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9(1):77–102. https://doi.org/10.1007/s13167-018-0128-8.
Moris D, Georgopoulos S, Felekouras E, Patsouris E, Theocharis S. The effect of endocannabinoid system in ischemia-reperfusion injury: a friend or a foe? Expert Opin Ther Targets. 2015;19(9):1261–75. https://doi.org/10.1517/14728222.2015.1043268.
Breivogel CS, Selley DE, Childers SR. Cannabinoid receptor agonist efficacy for stimulating [35S]GTPgammaS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J Biol Chem. 1998;273(27):16865–73. https://doi.org/10.1074/jbc.273.27.16865.
Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993;44(3):498–503.
Kellogg R, Mackie K, Straiker A. Cannabinoid CB1 receptor-dependent long-term depression in autaptic excitatory neurons. J Neurophysiol. 2009;102(2):1160–71. https://doi.org/10.1152/jn.00266.2009.
Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569(Pt 2):501–17. https://doi.org/10.1113/jphysiol.2005.091918.
Kenakin T. Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov. 2002;1(2):103–10. https://doi.org/10.1038/nrd722.
Kelley BG, Thayer SA. Delta 9-tetrahydrocannabinol antagonizes endocannabinoid modulation of synaptic transmission between hippocampal neurons in culture. Neuropharmacology. 2004;46(5):709–15. https://doi.org/10.1016/j.neuropharm.2003.11.005.
van Amsterdam J, Brunt T, van den Brink W. The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J Psychopharmacol. 2015;29(3):254–63. https://doi.org/10.1177/0269881114565142.
Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology. 2007;194(4):505–15. https://doi.org/10.1007/s00213-007-0861-5.
Tsou K, Patrick SL, Walker JM. Physical withdrawal in rats tolerant to delta 9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. Eur J Pharmacol. 1995;280(3):R13–5. https://doi.org/10.1016/0014-2999(95)00360-w.
Cecyre B, Thomas S, Ptito M, Casanova C, Bouchard JF. Evaluation of the specificity of antibodies raised against cannabinoid receptor type 2 in the mouse retina. Naunyn Schmiedeberg's Arch Pharmacol. 2014;387(2):175–84. https://doi.org/10.1007/s00210-013-0930-8.
Callen L, Moreno E, Barroso-Chinea P, Moreno-Delgado D, Cortes A, Mallol J, et al. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J Biol Chem. 2012;287(25):20851–65. https://doi.org/10.1074/jbc.M111.335273.
Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2019. https://doi.org/10.1038/s41582-019-0284-z.
Lopez G. Marijuana is legal for medical purposes in 33 states. 2019. https://www.vox.com/identities/2018/8/20/17938366/medical-marijuana-legalization-states-map. Accessed 22 November, 2019 2019.
Corroon J, Felice JF. The endocannabinoid system and its modulation by cannabidiol (CBD). Altern Ther Health Med. 2019;25(S2):6–14.
Simmerman E, Qin X, Yu JC, Baban B. Cannabinoids as a potential new and novel treatment for melanoma: a pilot study in a murine model. J Surg Res. 2019;235:210–5. https://doi.org/10.1016/j.jss.2018.08.055.
Baban B, Hoda N, Malik A, Khodadadi H, Simmerman E, Vaibhav K, et al. Impact of cannabidiol treatment on regulatory T-17 cells and neutrophil polarization in acute kidney injury. Am J Physiol Ren Physiol. 2018;315(4):F1149–F58. https://doi.org/10.1152/ajprenal.00112.2018.
Wang J, Wang Y, Tong M, Pan H, Li D. Medical cannabinoids for cancer cachexia: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:2864384. https://doi.org/10.1155/2019/2864384.
Thompson AE. JAMA Patient Page. Medical marijuana. JAMA. 2015;313(24):2508. https://doi.org/10.1001/jama.2015.6676.
Schatman ME. Medical use of cannabinoids. JAMA. 2015;314(16):1751. https://doi.org/10.1001/jama.2015.11435.
Hill KP, Hurley-Welljams-Dorof WM. Low to moderate quality evidence demonstrates the potential benefits and adverse events of cannabinoids for certain medical indications. Evid Based Med. 2016;21(1):17. https://doi.org/10.1136/ebmed-2015-110264.
Golubnitschaja O, Costigliola V. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14. https://doi.org/10.1186/1878-5085-3-14.
Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, et al. Brain cannabinoid CB(2) receptors modulate cocaine's actions in mice. Nat Neurosci. 2011;14(9):1160–6. https://doi.org/10.1038/nn.2874.
Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156(3):397–411. https://doi.org/10.1111/j.1476-5381.2008.00048.x.
Kaplan L, Klein T, Wilson M, Graves J. Knowledge, practices, and attitudes of Washington State health care professionals regarding medical cannabis. Cannabis Cannabinoid Res. 2019. https://doi.org/10.1089/can.2019.0051.
Goldenberg M, Reid MW, IsHak WW, Danovitch I. The impact of cannabis and cannabinoids for medical conditions on health-related quality of life: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;174:80–90. https://doi.org/10.1016/j.drugalcdep.2016.12.030.
Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. https://doi.org/10.1186/s13167-016-0072-4.
Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, et al. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;237(3):704–11. https://doi.org/10.1111/j.1432-1033.1996.0704p.x.
Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Mol Pharmacol. 1999;55(3):473–80.
Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–13.
Bonner TI. Molecular biology of cannabinoid receptors. J. Neuroimmunol. 1996;69(1–2):15–7.
Reggio PH. Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown. Curr Med Chem. 2010;17(14):1468–86. https://doi.org/10.2174/092986710790980005.
Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90(4):501–11. https://doi.org/10.1016/j.pbb.2008.05.010.
Onaivi ES, Ishiguro H, Gong J-P, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074(1):514–36. https://doi.org/10.1196/annals.1369.052.
Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071(1):10–23. https://doi.org/10.1016/j.brainres.2005.11.035.
Ishiguro H, Horiuchi Y, Ishikawa M, Koga M, Imai K, Suzuki Y, et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry. 2010;67(10):974–82. https://doi.org/10.1016/j.biopsych.2009.09.024.
Devane W, Hanus L, Breuer A, Pertwee R, Stevenson L, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science (New York, NY). 1992;258(5090):1946–9. https://doi.org/10.1126/science.1470919.
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202.
Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6(8):635–64.
Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, et al. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol. 2001;132(3):631–40. https://doi.org/10.1038/sj.bjp.0703850.
Sugiura T, Waku K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem Phys Lipids. 2000;108(1–2):89–106. https://doi.org/10.1016/s0009-3084(00)00189-4.
Childers SR, Breivogel CS. Cannabis and endogenous cannabinoid systems. Drug Alcohol Depend. 1998;51(1–2):173–87. https://doi.org/10.1016/s0376-8716(98)00075-1.
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. https://doi.org/10.1016/0006-2952(95)00109-D.
Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. 1999;274(5):2794–801.
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. https://doi.org/10.1006/bbrc.1995.2437.
Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, et al. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg Med Chem. 1999;7(12):2905–14. https://doi.org/10.1016/s0968-0896(99)00219-9.
Huffman JW, Bushell SM, Miller JR, Wiley JL, Martin BR. 1-Methoxy-, 1-deoxy-11-hydroxy- and 11-hydroxy-1-methoxy-Delta(8)-tetrahydrocannabinols: new selective ligands for the CB2 receptor. Bioorg Med Chem. 2002;10(12):4119–29. https://doi.org/10.1016/s0968-0896(02)00331-0.
Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48(3):443–50.
Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, et al. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol (Baltimore, MD : 1950). 2006;177(12):8796–805. https://doi.org/10.4049/jimmunol.177.12.8796.
Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, et al. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem. 2000;275(1):605–12. https://doi.org/10.1074/jbc.275.1.605.
Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29(4):225–32. https://doi.org/10.1016/j.tins.2006.01.008.
Pan X, Ikeda SR, Lewis DL. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol Pharmacol. 1996;49(4):707–14.
Gelfand EV, Cannon CP. Rimonabant: a cannabinoid receptor type 1 blocker for management of multiple cardiometabolic risk factors. J Am Coll Cardiol. 2006;47(10):1919–26. https://doi.org/10.1016/j.jacc.2005.12.067.
Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112(3):423–31. https://doi.org/10.1172/jci17725.
Pagotto U, Pasquali R. Fighting obesity and associated risk factors by antagonising cannabinoid type 1 receptors. Lancet (London, England). 2005;365(9468):1363–4. https://doi.org/10.1016/s0140-6736(05)66348-9.
Toguri JT, Moxsom R, Szczesniak AM, Zhou J, Kelly ME, Lehmann C. Cannabinoid 2 receptor activation reduces leukocyte adhesion and improves capillary perfusion in the iridial microvasculature during systemic inflammation. Clin Hemorheol Microcirc. 2015;61(2):237–49. https://doi.org/10.3233/ch-151996.
Rom S, Zuluaga-Ramirez V, Dykstra H, Reichenbach NL, Pacher P, Persidsky Y. Selective activation of cannabinoid receptor 2 in leukocytes suppresses their engagement of the brain endothelium and protects the blood-brain barrier. Am J Pathol. 2013;183(5):1548–58. https://doi.org/10.1016/j.ajpath.2013.07.033.
Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol. 2010;55(3):292–8. https://doi.org/10.1097/FJC.0b013e3181d2644d.
Fujii M, Sherchan P, Soejima Y, Doycheva D, Zhao D, Zhang JH. Cannabinoid receptor type 2 agonist attenuates acute neurogenic pulmonary edema by preventing neutrophil migration after subarachnoid hemorrhage in rats. Acta Neurochir Suppl. 2016;121:135–9. https://doi.org/10.1007/978-3-319-18497-5_24.
Braun M, Khan ZT, Khan MB, Kumar M, Ward A, Achyut BR, et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav Immun. 2018;68:224–37. https://doi.org/10.1016/j.bbi.2017.10.021.
Gentili M, Ronchetti S, Ricci E, Di Paola R, Gugliandolo E, Cuzzocrea S, et al. Selective CB2 inverse agonist JTE907 drives T cell differentiation towards a Treg cell phenotype and ameliorates inflammation in a mouse model of inflammatory bowel disease. Pharmacol Res. 2019;141:21–31. https://doi.org/10.1016/j.phrs.2018.12.005.
Kong W, Li H, Tuma RF, Ganea D. Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell Immunol. 2014;287(1):1–17. https://doi.org/10.1016/j.cellimm.2013.11.002.
Hasko J, Fazakas C, Molnar J, Nyul-Toth A, Herman H, Hermenean A, et al. CB2 receptor activation inhibits melanoma cell transmigration through the blood-brain barrier. Int J Mol Sci. 2014;15(5):8063–74. https://doi.org/10.3390/ijms15058063.
Sanchez MG, Ruiz-Llorente L, Sanchez AM, Diaz-Laviada I. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1) and CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell Signal. 2003;15(9):851–9. https://doi.org/10.1016/s0898-6568(03)00036-6.
Guzman M, Galve-Roperh I, Sanchez C. Ceramide: a new second messenger of cannabinoid action. Trends Pharmacol Sci. 2001;22(1):19–22. https://doi.org/10.1016/s0165-6147(00)01586-8.
Acknowledgments
The authors thank Colby Zahn for the illustration provided in this review.
Funding
Financial support for this study was provided by grants from the National Institutes of Neurological Diseases and Stroke (NS065172, NS097825 to KMD and NS110378 to BB/KMD), National Institutes of Child Health and Development (HD094606 to KV), and American Heart Association (GRNT33700286 to KMD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reddy, V., Grogan, D., Ahluwalia, M. et al. Targeting the endocannabinoid system: a predictive, preventive, and personalized medicine-directed approach to the management of brain pathologies. EPMA Journal 11, 217–250 (2020). https://doi.org/10.1007/s13167-020-00203-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-020-00203-4
Keywords
- Endocannabinoid system
- Anandamide
- 2-Arachidonyl glycerol
- Cannabinoid receptor
- Neurological disorder
- Traumatic brain injury
- Alzheimer’s disease
- Parkinson’s disease
- Amyloid lateral sclerosis
- Cancer
- Pain
- Epilepsy
- Multiple sclerosis
- Huntington’s disease
- Schizophrenia
- Stroke
- Anxiety
- Depression
- Multi-professional expertise
- Therapeutic strategies
- Health policy
- Disease management
- Predictive preventive personalized medicine (PPPM)