Abstract
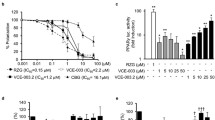
Different plant-derived and synthetic cannabinoids have shown to be neuroprotective in experimental models of Huntington’s disease (HD) through cannabinoid receptor-dependent and/or independent mechanisms. Herein, we studied the effects of cannabigerol (CBG), a nonpsychotropic phytocannabinoid, in 2 different in vivo models of HD. CBG was extremely active as neuroprotectant in mice intoxicated with 3-nitropropionate (3NP), improving motor deficits and preserving striatal neurons against 3NP toxicity. In addition, CBG attenuated the reactive microgliosis and the upregulation of proinflammatory markers induced by 3NP, and improved the levels of antioxidant defenses that were also significantly reduced by 3NP. We also investigated the neuroprotective properties of CBG in R6/2 mice. Treatment with this phytocannabinoid produced a much lower, but significant, recovery in the deteriorated rotarod performance typical of R6/2 mice. Using HD array analysis, we were able to identify a series of genes linked to this disease (e.g., symplekin, Sin3a, Rcor1, histone deacetylase 2, huntingtin-associated protein 1, δ subunit of the gamma-aminobutyric acid-A receptor (GABA-A), and hippocalcin), whose expression was altered in R6/2 mice but partially normalized by CBG treatment. We also observed a modest improvement in the gene expression for brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), and peroxisome proliferator-activated receptor-γ (PPARγ), which is altered in these mice, as well as a small, but significant, reduction in the aggregation of mutant huntingtin in the striatal parenchyma in CBG-treated animals. In conclusion, our results open new research avenues for the use of CBG, alone or in combination with other phytocannabinoids or therapies, for the treatment of neurodegenerative diseases such as HD.











Similar content being viewed by others
References
Roze E, Bonnet C, Betuing S, Caboche J. Huntington's disease. Adv Exp Med Biol 2010;685:45–63.
Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev 2010;90:905–981.
Johnson CD, Davidson BL. Huntington's disease: progress toward effective disease-modifying treatments and a cure. Hum Mol Genet 2010;19:R98-R102.
Pazos MR, Sagredo O, Fernández-Ruiz J. The endocannabinoid system in Huntington's disease. Curr Pharm Des 2008;14:2317–2325.
Fernández-Ruiz J, García C, Sagredo O, Gómez-Ruiz M, de Lago E. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets 2010;14:387–404.
Lastres-Becker I, Bizat N, Boyer F, Hantraye P, Fernández-Ruiz J, Brouillet E. Potential involvement of cannabinoid receptors in 3-nitropropionic acid toxicity in vivo. Neuroreport 2004;15:2375–2379.
Sagredo O, Ramos JA, Decio A, Mechoulam R, Fernández-Ruiz J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur J Neurosci 2007;26:843–851.
Blázquez C, Chiarlone A, Sagredo O, et al. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington's disease. Brain 2011;134:119–136.
Palazuelos J, Aguado T, Pazos MR, et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain 2009;132:3152–3164.
Sagredo O, González S, Aroyo I, et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for Huntington's disease. Glia 2009;57:1154–1167.
Pintor A, Tebano MT, Martire A, et al. The cannabinoid receptor agonist WIN 55,212-2 attenuates the effects induced by quinolinic acid in the rat striatum. Neuropharmacology 2006;51:1004–1012.
Sastre-Garriga J, Vila C, Clissold S, Montalban X. THC and CBD oromucosal spray (Sativex®) in the management of spasticity associated with multiple sclerosis. Expert Rev Neurother 2011;11:627–637.
Zajicek JP, Apostu VI. Role of cannabinoids in multiple sclerosis. CNS Drugs 2011;25:187–201.
Sagredo O, Pazos MR, Satta V, Ramos JA, Pertwee RG, Fernández-Ruiz J. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington's disease. J Neurosci Res 2011;89:1509–1518.
Fernández-Ruiz J, Sagredo O, Pazos MR, et al. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol 2013;75:323–333.
O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol 2007;152:576–582.
Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 2009;30:515–527.
Hill AJ, Williams CM, Whalley BJ, Stephens GJ. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther 2012;133:79–97.
Granja AG, Carrillo-Salinas F, Pagani A, et al. A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J Neuroimmune Pharmacol 2012;7:1002–1016.
Borrelli F, Fasolino I, Romano B, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol 2013;85:1306–1316.
Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol 2010;159:129–141.
Brouillet E, Jacquard C, Bizat N, Blum D. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington's disease. J Neurochem 2005;95:1521–1540.
Pouladi MA, Xie Y, Skotte NH, et al. Full-length huntingtin levels modulate body weight by influencing insulin-like growth factor 1 expression. Hum Mol Genet 2010;19:1528–1538.
Fernagut PO, Diguet E, Stefanova N, et al. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience 2002;114:1005–1017.
Luck H. Catalase. In: Bergmeyer HU (ed.) Methods of enzyme analysis. Academic Press, New York, 1971, p. 885.
Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and and assay for superoxide dismutase. Arch Biochem Biophys 1978;186:189–195.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1978;82:70–77.
Gray M, Shirasaki DI, Cepeda C, et al. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci 2008;28:6182–6195.
Alvarez FJ, Lafuente H, Rey-Santano MC, et al. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr Res 2008;64:653–658.
Trettel F, Rigamonti D, Hilditch-Maguire P, et al. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet 2000;9:2799–2809.
Rangone H, Poizat G, Troncoso J, et al. The serum- and glucocorticoid-induced kinase SGK inhibits mutant huntingtin-induced toxicity by phosphorylating serine 421 of huntingtin. Eur J Neurosci 2004;19:273–279.
Tuohy TM, Wallingford N, Liu Y, et al. CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation-independent demyelination and dysmyelination. Glia 2004;47:335–345.
Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington's disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington's disease. Neuroscience 2000;97:505–519.
Battista N, Bari M, Tarditi A, et al. Severe deficiency of the fatty acid amide hydrolase (FAAH) activity segregates with the Huntington's disease mutation in peripheral lymphocytes. Neurobiol Dis 2007;27:108–116.
Apostol BL, Simmons DA, Zuccato C, et al. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol Cell Neurosci 2008;39:8–20.
Estrada-Sánchez AM, Montiel T, Segovia J, Massieu L. Glutamate toxicity in the striatum of the R6/2 Huntington's disease transgenic mice is age-dependent and correlates with decreased levels of glutamate transporters. Neurobiol Dis 2009;34:78–86.
Luthi-Carter R, Strand A, Peters NL, et al. Decreased expression of striatal signaling genes in a mouse model of Huntington's disease. Hum Mol Genet 2000;9:1259–1271.
Crocker SF, Costain WJ, Robertson HA. DNA microarray analysis of striatal gene expression in symptomatic transgenic Huntington's mice (R6/2) reveals neuroinflammation and insulin associations. Brain Res 2006;1088:176–186.
Deckel AW, Gordinier A, Nuttal D, et al. Reduced activity and protein expression of NOS in R6/2 HD transgenic mice: effects of L-NAME on symptom progression. Brain Res 2001;919:70–81.
Yu ZX, Li SH, Evans J, Pillarisetti A, Li H, Li XJ. Mutant huntingtin causes context-dependent neurodegeneration in mice with Huntington's disease. J Neurosci 2003;23:2193–2202.
Zucker B, Luthi-Carter R, Kama JA, et al. Transcriptional dysregulation in striatal projection- and interneurons in a mouse model of Huntington's disease: neuronal selectivity and potential neuroprotective role of HAP1. Hum Mol Genet 2005;14:179–189.
Jovicic A, Zaldivar Jolissaint JF, Moser R, Silva Santos Mde F, Luthi-Carter R. MicroRNA-22 (miR-22) overexpression is neuroprotective via general anti-apoptotic effects and may also target specific Huntington's disease-related mechanisms. PLOS ONE 2013;8:e54222.
Mielcarek M, Benn CL, Franklin SA, et al. SAHA decreases HDAC 2 and 4 levels in vivo and improves molecular phenotypes in the R6/2 mouse model of Huntington's disease. PLOS ONE 2011;6:e27746.
Rudinskiy N, Kaneko YA, Beesen AA, et al. Diminished hippocalcin expression in Huntington's disease brain does not account for increased striatal neuron vulnerability as assessed in primary neurons. J Neurochem 2009;111:460–472.
Consroe P, Laguna J, Allender J, et al. Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacol Biochem Behav 1991;40:701–708.
Curtis A, Rickards H. Nabilone could treat chorea and irritability in Huntington's disease. J Neuropsychiatry Clin Neurosci 2006;18:553–554.
Curtis A, Mitchell I, Patel S, Ives N, Rickards H. A pilot study using nabilone for symptomatic treatment in Huntington's disease. Mov Disord 2009;24:2254–2259.
Müller-Vahl KR, Schneider U, Emrich HM. Nabilone increases choreatic movements in Huntington's disease. Mov Disord 1999;14:1038–1040.
Valdeolivas S, Satta V, Pertwee RG, Fernández-Ruiz J, Sagredo O. Sativex-like combination of phytocannabinoids is neuroprotective in malonate-lesioned rats, an inflammatory model of Huntington's disease: role of CB1 and CB2 receptors. ACS Chem Neurosci 2012;3:400–406.
Deiana S, Watanabe A, Yamasaki Y, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl) 2012;219:859–873.
Bode FJ, Stephan M, Wiehager S, et al. Increased numbers of motor activity peaks during light cycle are associated with reductions in adrenergic alpha(2)-receptor levels in a transgenic Huntington's disease rat model. Behav Brain Res 2009;205:175–182.
Martel J, Chopin P, Colpaert F, Marien M. Neuroprotective effects of the alpha2-adrenoceptor antagonists, (+)-efaroxan and (+/−)-idazoxan, against quinolinic acid-induced lesions of the rat striatum. Exp Neurol 1998;154:595–601.
Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GV. Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin-expressing cells: possible role of peroxisome proliferator-activated receptor-γ (PPAR-γ) in the pathogenesis of Huntington disease. J Biol Chem 2008;283:25628–25637.
Johri A, Calingasan NY, Hennessey TM, et al. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington's disease. Hum Mol Genet 2012;21:1124–1137.
Jin J, Albertz J, Guo Z, et al. Neuroprotective effects of PPAR-γ agonist rosiglitazone in N171-82Q mouse model of Huntington's disease. J Neurochem 2013;125:410–419.
Schütz B, Reimann J, Dumitrescu-Ozimek L, et al. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J Neurosci 2005;25:7805–7812.
Strum JC, Shehee R, Virley D, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis 2007;11:45–51.
Strand AD, Baquet ZC, Aragaki AK, et al. Expression profiling of Huntington's disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci 2007;27:11758–11768.
Acknowledgments
This work was supported by grants from CIBERNED (CB06/05/0089), MICINN (SAF2009/11847), and CAM (S2011/BMD-2308) to O.S. and S.V., and from MICINN (SAF2010/19292) and MINECO (IPT-2011-0861-900000) to E.M. These agencies had no further role in study design, the collection, analysis and interpretation of data, in the writing of the report, nor in the decision to submit the paper for publication. S.V. was supported by the Complutense University-Predoctoral Program. We are indebted to Yolanda García-Movellán for administrative assistance.
Conflict of interest
C.N. and M.L.B. are employees of VivaCell Biotechnology Spain and they were supported by MINECO IPT-2011-0861-900000 and FEDER-INTERCONNECTA ITC-20111029 grants to VivaCell Biotechnology.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
S. Valdeolivas and C. Navarrete share first authorship.
E. Muñoz and O. Sagredo share senior authorship of this study.
Rights and permissions
About this article
Cite this article
Valdeolivas, S., Navarrete, C., Cantarero, I. et al. Neuroprotective Properties of Cannabigerol in Huntington’s Disease: Studies in R6/2 Mice and 3-Nitropropionate-lesioned Mice. Neurotherapeutics 12, 185–199 (2015). https://doi.org/10.1007/s13311-014-0304-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-014-0304-z