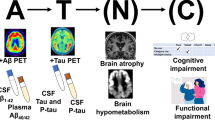
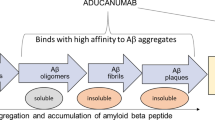
Abstract
Alzheimer’s disease (AD) is the most common cause of dementia, which is characterized by a progressive neurodegenerative disorder that is extremely difficult to treat and severely reduces quality of life. Amyloid beta (Aβ) has been the primary target of experimental therapies owing to the neurotoxicity of Aβ and the brain Aβ load detected in humans by amyloid positron emission tomography (PET) imaging. Recently completed phase 2 and 3 trials of third-generation anti-amyloid immunotherapies indicated clinical efficacy in significantly reducing brain Aβ load and inhibiting the progression of cognitive decline. Anti-amyloid immunotherapies are the first effective disease-modifying therapies for AD, and aducanumab and lecanemab were recently approved through the US Food and Drug Administration’s accelerated approval pathway. However, these therapies still exhibit insufficient clinical efficacy and are associated with amyloid-related imaging abnormalities. Further advances in the field of AD therapeutics are required to revolutionize clinical AD treatment, dementia care, and preventive cognitive healthcare.


Similar content being viewed by others
Data Availability
For this type of study, data sharing is not applicable as no datasets were generated or analyzed.
References
Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer report–the global impact of dementia; 2015
Cummings J, Lee G, Nahed P, Kambar MEZN, Zhong K, Fonseca J, et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement (N Y). 2022;8:e12295. https://doi.org/10.1002/trc2.12295.
Klein G, Delmar P, Voyle N, Rehal S, Hofmann C, Abi-Saab D, et al. Gantenerumab reduces amyloid-β plaques in patients with prodromal to moderate Alzheimer’s disease: a PET substudy interim analysis. Alzheimers Res Ther. 2019;11:101. https://doi.org/10.1186/s13195-019-0559-z.
Lowe SL, Duggan Evans C, Shcherbinin S, Cheng YJ, Willis BA, Gueorguieva I, et al. Donanemab (LY3002813) phase 1b study in Alzheimer’s disease: rapid and sustained reduction of brain amyloid measured by florbetapir F18 imaging. J Prev Alzheimers Dis. 2021;8:414–24. https://doi.org/10.14283/jpad.2021.56.
Panza F, Lozupone M, Solfrizzi V, Sardone R, Piccininni C, Dibello V, et al. BACE inhibitors in clinical development for the treatment of Alzheimer’s disease. Expert Rev Neurother. 2018;18:847–57. https://doi.org/10.1080/14737175.2018.1531706.
Viola KL, Bicca MA, Bebenek AM, Kranz DL, Nandwana V, Waters EA, et al. The therapeutic and diagnostic potential of amyloid beta oligomers selective antibodies to treat Alzheimer’s disease. Front Neurosci. 2021;15:768646. https://doi.org/10.3389/fnins.2021.768646.
De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, et al. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol Aging. 2008;29:1334–47. https://doi.org/10.1016/j.neurobiolaging.2007.02.029.
Rudinskiy N, Fuerer C, Demurtas D, Zamorano S, De Piano C, Herrmann AG, et al. Amyloid-beta oligomerization is associated with the generation of a typical peptide fragment fingerprint. Alzheimers Dement. 2016;12:996–1013. https://doi.org/10.1016/j.jalz.2016.03.011.
Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14:2013–54. https://doi.org/10.1089/ars.2010.3208.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–19. https://doi.org/10.1002/ana.20009.
Wang J, Jin C, Zhou J, Zhou R, Tian M, Lee HJ, et al. PET molecular imaging for pathophysiological visualization in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2023;50:765–83. https://doi.org/10.1007/s00259-022-05999-z.
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. https://doi.org/10.1126/science.166067.
Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. https://doi.org/10.15252/emmm.201606210.
Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 2014;84:608–22. https://doi.org/10.1016/j.neuron.2014.10.038.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. https://doi.org/10.1016/j.jalz.2018.02.018.
Wilcock DM, Colton CA. Anti-amyloid-β immunotherapy in Alzheimer’s disease: relevance of transgenic mouse studies to clinical trials. J Alzheimers Dis. 2008;15:555–69. https://doi.org/10.3233/jad-2008-15404.
Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003;61:46–54. https://doi.org/10.1212/01.wnl.0000073623.84147.a8.
Lacosta AM, Pascual-Lucas M, Pesini P, Casabona D, Pérez-Grijalba V, Marcos-Campos I, et al. Safety, tolerability and immunogenicity of an active anti-Abeta (40) vaccine (ABvac40) in patients with Alzheimer’s disease: a randomised, doubleblind, placebo-controlled, phase I trial. Alzheimers Res Ther. 2018;10:12. https://doi.org/10.1186/s13195-018-0340-8.
Wang CY, Wang PN, Chiu MJ, Finstad CL, Lin F, Lynn S, et al. UB-311, a novel UBITh®amyloid beta peptide vaccine for mild Alzheimer’s disease. Alzheimers Dement (N Y). 2017;3:262–72. https://doi.org/10.1016/j.trci.2017.03.005.
Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, et al. Passive immunotherapy against Aβ in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. https://doi.org/10.1186/1742-2094-1-24.
Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–8. https://doi.org/10.1073/pnas.0436286100.
Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, et al. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004;24:6144–51. https://doi.org/10.1523/JNEUROSCI.1090-04.2004.
Hartman RE, Izumi Y, Bales KR, Paul SM, Wozniak DF, Holtzman DM. Treatment with an amyloid-β antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25:6213–20. https://doi.org/10.1523/JNEUROSCI.0664-05.2005.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of Bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–33. https://doi.org/10.1056/NEJMoa1304839.
Chapleau M, Iaccarino L, Soleimani-Meigooni D, Rabinovici GD. The role of amyloid PET in imaging neurodegenerative disorders: a review. J Nucl Med. 2022;63;Suppl 1:13S–9S–S19. https://doi.org/10.2967/jnumed.121.263195
Lendel C, Bjerring M, Dubnovitsky A, Kelly RT, Filippov A, Antzutkin ON, et al. A hexameric peptide barrel as building block of amyloid-β protofibrils. Angew Chem Int Ed Engl. 2014;53:12756–60. https://doi.org/10.1002/anie.201406357.
Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med. 2018;378:321–30. https://doi.org/10.1056/NEJMoa1705971.
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–21. https://doi.org/10.1056/NEJMoa1312889.
Meilandt W, Maloney J, Imperio J, Bainbridge T, Reichelt M, Mandikian D, et al. Characterization of the selective in vivo and in vitro binding properties of crenezumab: insights into crenezumab’s unique mechanism of action. Alzheimers Res Ther. 2019;11:97. https://doi.org/10.1186/s13195-019-0553-5.
Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Cho W, et al. ABBY: a phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology. 2018;90:e1889–97. https://doi.org/10.1212/WNL.0000000000005550.
Ostrowitzki S, Bittner T, Sink KM, Mackey H, Rabe C, Honig LS, et al. Evaluating the safety and efficacy of crenezumab vs placebo in adults with early Alzheimer disease: two phase 3 randomized placebo-controlled trials. JAMA Neurol. 2022;79:1113–21. https://doi.org/10.1001/jamaneurol.2022.2909.
Reiman EM, Pruzin JJ, Rios-Romenets S, Brown C, Giraldo M, Acosta-Baena N, et al. A public resource of baseline data from the Alzheimer’s prevention initiative autosomal-dominant Alzheimer’s disease trial. Alzheimers Dement. 2023;19:1938–46. https://doi.org/10.1002/alz.12843.
Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther. 2017;9:95. https://doi.org/10.1186/s13195-017-0318-y.
Bateman RJ, Cummings J, Schobel S, Salloway S, Vellas B, Boada M, et al. Gantenerumab: an anti-amyloid monoclonal antibody with potential disease-modifying effects in early Alzheimer’s disease. Alzheimers Res Ther. 2022;14:178. https://doi.org/10.1186/s13195-022-01110-8.
Roche announces results from the GRADUATE Phase III trials of gantenerumab; 2022. https://www.roche.com/media/releases/med-cor-2022-11-14. Accessed 11/08/2023
Arndt JW, Qian F, Smith BA, Quan C, Kilambi KP, Bush MW, et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci Rep. 2018;8:6412. https://doi.org/10.1038/s41598-018-24501-0.
Colvin MT, Silvers R, Ni QZ, Can TV, Sergeyev I, Rosay M, et al. Atomic resolution structure of monomorphic Aβ42 amyloid fibrils. J Am Chem Soc. 2016;138:9663–74. https://doi.org/10.1021/jacs.6b05129.
Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–6. https://doi.org/10.1038/nature19323.
Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron. 2013;79:887–902. https://doi.org/10.1016/j.neuron.2013.06.036.
Budd Haeberlein S, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197–210. https://doi.org/10.14283/jpad.2022.30.
Salloway S, Chalkias S, Barkhof F, Burkett P, Barakos J, Purcell D, et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79:13–21. https://doi.org/10.1001/jamaneurol.2021.4161.
Beshir SA, Aadithsoorya AM, Parveen A, Goh SSL, Hussain N, Menon VB. Aducanumab therapy to treat Alzheimer’s disease: a narrative review. Int J Alzheimers Dis. 2022;2022:9343514. https://doi.org/10.1155/2022/9343514.
Basun H, Bogdanovic N, Ingelsson M, Almkvist O, Näslund J, Axelman K, et al. Clinical and neuropathological features of the arctic APP gene mutation causing early-onset Alzheimer disease. Arch Neurol. 2008;65:499–505. https://doi.org/10.1001/archneur.65.4.499.
Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced abeta protofibril formation. Nat Neurosci. 2001;4:887–93. https://doi.org/10.1038/nn0901-887.
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. https://doi.org/10.1056/NEJMoa2212948.
Papp KV, Rentz DM, Orlovsky I, Sperling RA, Mormino EC. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: the PACC5. Alzheimers Dement (N Y). 2017;3:668–77. https://doi.org/10.1016/j.trci.2017.10.004.
Jawhar S, Wirths O, Bayer TA. Pyroglutamate amyloid-beta (Aβ): a hatchet man in Alzheimer disease. J Biol Chem. 2011;286:38825–32. https://doi.org/10.1074/jbc.R111.288308.
Iwatsubo T, Saido TC, Mann DM, Lee VM, Trojanowski JQ. Full-length amyloid-beta (1–42 (43)) and amino-terminally modified and truncated amyloid-beta 42 (43) deposit in diffuse plaques. Am J Pathol. 1996;149:1823–30.
Demattos RB, Lu J, Tang Y, Racke MM, Delong CA, Tzaferis JA, et al. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron. 2012;76:908–20. https://doi.org/10.1016/j.neuron.2012.10.029.
Espay AJ. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;385:666–7. https://doi.org/10.1056/NEJMc2109455.
Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384:1691–704. https://doi.org/10.1056/NEJMoa2100708.
Shcherbinin S, Andersen SW, Evans CD, Lo AC, Lu M, Navitsky M, et al. TRAILBLAZER-ALZ Study: dynamics of amyloid reduction after donanemab treatment. Alzheimers Dement. 2021;17:e057492. https://doi.org/10.1002/alz.057492.
Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330:512–27. https://doi.org/10.1001/jama.2023.13239.
Jin Y. Safety and amyloid plaque reduction effects of remternetug in patients with Alzheimer’s disease: interim analysis from a phase 1 study AD/PD Conference. Sweden: Gothernburg; 2023. p. 2023.
Siemers E, Hitchcock J, Sundell K, Dean R, Jerecic J, Cline E, et al. ACU193, A monoclonal antibody that selectively binds soluble Aβ oligomers: development rationale, phase 1 trial design, and clinical development plan. J Prev Alzheimers Dis. 2023;10:19–24. https://doi.org/10.14283/jpad.2022.93.
ALZFORUM. Trontinemab: AlzForum Foundation Inc.; 2023. https://www.alzforum.org/therapeutics/trontinemab. Accessed 11/08/2023
Yadollahikhales G, Rojas JC. Anti-amyloid immunotherapies for Alzheimer’s disease: a 2023 clinical update. Neurotherapeutics. 2023;20:914–31. https://doi.org/10.1007/s13311-023-01405-0.
Acknowledgements
The author would like to thank the neurology and nuclear medicine staffs at Dong-A University College of Medicine.
Author information
Authors and Affiliations
Contributions
Kyung Won Park contributed to the conceptualization, design, and writing of this review manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Kyung Won Park declares no competing interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
For this type of study, formal consent is not required and informed consent is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, K.W. Anti-amyloid Antibody Therapies for Alzheimer’s Disease. Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s13139-024-00848-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13139-024-00848-3