Abstract
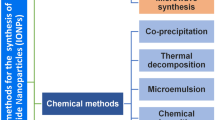
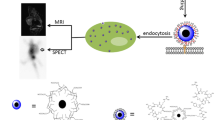
Supermagnetic Iron Oxide Nanoparticles (SPIONs) are nanoparticles that have an iron oxide core and a functionalized shell. SPIONs have recently raised much interest in the scientific community, given their exciting potential diagnostic and theragnostic applications. The possibility to modify their surface and the characteristics of their core make SPIONs a specific contrast agent for magnetic resonance imaging but also an intriguing family of tracer for nuclear medicine. An example is 68Ga-radiolabeled bombesin-conjugated to superparamagnetic nanoparticles coated with trimethyl chitosan that is selective for the gastrin-releasing peptide receptors. These receptors are expressed by several human cancer cells such as breast and prostate neoplasia. Since the coating does not interfere with the properties of the molecules bounded to the shell, it has been proposed to link SPIONs with antibodies. SPIONs can be used also to monitor the biodistribution of mesenchymal stromal cells and take place in various applications. The aim of this review of literature is to analyze the diagnostic aspect of SPIONs in magnetic resonance imaging and in nuclear medicine, with a particular focus on sentinel lymph node applications. Moreover, it is taken into account the possible toxicity and the effects on human physiology to determine the SPIONs’ safety.




Similar content being viewed by others
References
Lassenberger A, Scheberl A, Stadlbauer A, Stiglbauer A, Helbich T, Reimhult E. Individually stabilized, Superparamagnetic nanoparticles with controlled shell and size leading to exceptional stealth properties and high relaxivities. ACS Appl Mater Interfaces. 2017;9:3343–53.
Uthaman S, Lee SJ, Cherukula K, Cho CS, Park IK. Polysaccharide-coated magnetic nanoparticles for imaging and gene therapy. Biomed Res Int. 2015;2015:959175.
Liu F, Le W, Mei T, Wang T, Chen L, Lei Y, et al. In vitro and in vivo targeting imaging of pancreatic cancer using a Fe3O4@SiO2 nanoprobe modified with anti-mesothelin antibody. Int J Nanomedicine. 2016;11:2195–207.
Tomanek B, Iqbal U, Blasiak B, Abulrob A, Albaghdadi H, Matyas JR, et al. Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging. Neuro-Oncology. 2012;14:53–63.
Yang RM, Fu CP, Fang JZ, Xu XD, Wei XH, Tang WJ, et al. Hyaluronan-modified superparamagnetic iron oxide nanoparticles for bimodal breast cancer imaging and photothermal therapy. Int J Nanomedicine. 2017;12:197–206.
Dulinska-Litewka J, Lazarczyk A, Halubiec P, Szafranski O, Karnas K, Karewicz A. Superparamagnetic Iron Oxide Nanoparticles-Current and Prospective Medical Applications. Materials (Basel). 2019;12(4).
Markides H, Newell KJ, Rudorf H, Ferreras LB, Dixon JE, Morris RH, et al. Ex vivo MRI cell tracking of autologous mesenchymal stromal cells in an ovine osteochondral defect model. Stem Cell Res Ther. 2019;10:25.
Sciarra A, Gentilucci A, Silvestri I, Salciccia S, Cattarino S, Scarpa S, et al. Androgen receptor variant 7 (AR-V7) in sequencing therapeutic agents for castratrion resistant prostate cancer: a critical review. Medicine (Baltimore). 2019;98:e15608.
Ricci M, Frantellizzi V, Bulzonetti N, De Vincentis G. Reversibility of castration resistance status after radium-223 dichloride treatment: clinical evidence and review of the literature. Int J Radiat Biol. 2018:1–29.
Winter A, Kowald T, Paulo TS, Goos P, Engels S, Gerullis H, et al. Magnetic resonance sentinel lymph node imaging and magnetometer-guided intraoperative detection in prostate cancer using superparamagnetic iron oxide nanoparticles. Int J Nanomedicine. 2018;13:6689–98.
Stanik M, Macik D, Capak I, Mareckova N, Lzicarova E, Dolezel J. Sentinel lymph node dissection in prostate cancer using superparamagnetic particles of iron oxide: early clinical experience. Int Urol Nephrol. 2018;50:1427–33.
Mehralivand S, van der Poel H, Winter A, Choyke PL, Pinto PA, Turkbey B. Sentinel lymph node imaging in urologic oncology. Transl Androl Urol. 2018;7:887–902.
Karakatsanis A, Daskalakis K, Stalberg P, Olofsson H, Andersson Y, Eriksson S, et al. Superparamagnetic iron oxide nanoparticles as the sole method for sentinel node biopsy detection in patients with breast cancer. Br J Surg. 2017;104:1675–85.
Mekseriwattana W, Srisuk S, Kriangsaksri R, Niamsiri N, Prapainop K. The impact of serum proteins and surface chemistry on magnetic nanoparticle colloidal stability and cellular uptake in breast cancer cells. AAPS PharmSciTech. 2019;20:55.
Zhang L, Jin R, Sun R, Du L, Liu L, Zhang K, et al. Superparamagnetic iron oxide nanoparticles as magnetic resonance imaging contrast agents and induced autophagy response in endothelial progenitor cells. J Biomed Nanotechnol. 2019;15:396–404.
Singh N, Jenkins GJ, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nanotechnol Rev. 2010;1.
Patil RM, Thorat ND, Shete PB, Bedge PA, Gavde S, Joshi MG, et al. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem Biophys Rep. 2018;13:63–72.
Ansari MO, Ahmad MF, Shadab GGHA, Siddique HR. Superparamagnetic iron oxide nanoparticles based cancer theranostics: a double edge sword to fight against cancer. J Drug Delivery Sci Technol. 2018;45:177–83.
Elias A, Tsourkas A. Imaging circulating cells and lymphoid tissues with iron oxide nanoparticles. Hematology Am Soc Hematol Educ Program. 2009:720–6.
Kumar P, Agnihotri S. Synthesis of dox drug conjugation and citric acid stabilized superparamagnetic iron-oxide nanoparticles for drug delivery. Biochem Physiol: Open Access. 2016;01.
Wu VM, Huynh E, Tang S, Uskokovic V. Brain and bone cancer targeting by a ferrofluid composed of superparamagnetic iron-oxide/silica/carbon nanoparticles (earthicles). Acta Biomater. 2019;88:422–47.
Awada H, Al Samad A, Laurencin D, Gilbert R, Dumail X, El Jundi A, et al. Controlled anchoring of iron oxide nanoparticles on polymeric nanofibers: easy access to core@shell organic-inorganic nanocomposites for magneto-scaffolds. ACS Appl Mater Interfaces. 2019;11:9519–29.
Unterweger H, Janko C, Schwarz M, Dezsi L, Urbanics R, Matuszak J, et al. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: a biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int J Nanomedicine. 2017;12:5223–38.
Szpak A, Kania G, Skorka T, Tokarz W, Zapotoczny S, Nowakowska M. Stable aqueous dispersion of superparamagnetic iron oxide nanoparticles protected by charged chitosan derivatives. J Nanopart Res. 2013;15:1372.
Thomas RG, Moon MJ, Lee H, Sasikala AR, Kim CS, Park IK, et al. Hyaluronic acid conjugated superparamagnetic iron oxide nanoparticle for cancer diagnosis and hyperthermia therapy. Carbohydr Polym. 2015;131:439–46.
Zhao L, Chano T, Morikawa S, Saito Y, Shiino A, Shimizu S, et al. Hyperbranched polyglycerol-grafted superparamagnetic iron oxide nanoparticles: synthesis, characterization, functionalization, size separation, magnetic properties, and biological applications. Adv Funct Mater. 2012;22:5107–17.
Prabhu S, Mutalik S, Rai S, Udupa N, Rao BSS. PEGylation of superparamagnetic iron oxide nanoparticle for drug delivery applications with decreased toxicity: an in vivo study. J Nanopart Res. 2015;17.
Thapa B, Diaz-Diestra D, Beltran-Huarac J, Weiner BR, Morell G. Enhanced MRI T 2 relaxivity in contrast-probed anchor-free PEGylated iron oxide nanoparticles. Nanoscale Res Lett. 2017;12:312.
Kurtan U, Esir S, Baykal A, Sözeri H. Poly(amidoamine)-grafted superparamagnetic iron oxide nanoparticles: synthesis and characterization. J Supercond Nov Magn. 2014;27:2097–103.
Zhang P, Qiao Y, Wang C, Ma L, Su M. Enhanced radiation therapy with internalized polyelectrolyte modified nanoparticles. Nanoscale. 2014;6:10095–9.
Huang S-J, Ke J-H, Chen G-J, Wang L-F. One-pot synthesis of PDMAEMA-bound iron oxide nanoparticles for magnetofection. J Mater Chem B. 2013;1:5916.
Kurzhals S, Pretzner B, Reimhult E, Zirbs R. Thermoresponsive polypeptoid-coated superparamagnetic iron oxide nanoparticles by surface-initiated polymerization. Macromol Chem Phys. 2017;218:1700116.
Sulek S, Mammadov B, Mahcicek DI, Sozeri H, Atalar E, Tekinay AB, et al. Peptide functionalized superparamagnetic iron oxide nanoparticles as MRI contrast agents. J Mater Chem. 2011;21:15157–62.
Amiri H, Saeidi K, Borhani P, Manafirad A, Ghavami M, Zerbi V. Alzheimer's disease: pathophysiology and applications of magnetic nanoparticles as MRI theranostic agents. ACS Chem Neurosci. 2013;4:1417–29.
Wang Y, Ye F, Jeong EK, Sun Y, Parker DL, Lu ZR. Noninvasive visualization of pharmacokinetics, biodistribution and tumor targeting of poly[N-(2-hydroxypropyl)methacrylamide] in mice using contrast enhanced MRI. Pharm Res. 2007;24:1208–16.
Kowalchuk RM, Pollack SR, Corcoran TA. Zeta potential of bone from particle electrophoresis: solution composition and kinetic effects. J Biomed Mater Res. 1995;29:47–57.
Al Mahrouqi D, Vinogradov J, Jackson MD. Zeta potential of artificial and natural calcite in aqueous solution. Adv Colloid Interf Sci. 2017;240:60–76.
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63:24–46.
Sun ZX, Su FW, Forsling W, Samskog PO. Surface characteristics of magnetite in aqueous suspension. J Colloid Interface Sci. 1998;197:151–9.
Teja AS, Koh PY. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog Cryst Growth Charact Mater. 2009;55:22–45.
Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021.
Shao C, Liu F, Le W, Mei T, Wang T, Chen L, et al. In vitro and in vivo targeting imaging of pancreatic cancer using a Fe3O4@SiO2 nanoprobe modified with anti-mesothelin antibody. Int J Nanomedicine. 2016;2195.
Mahajan S, Koul V, Choudhary V, Shishodia G, Bharti AC. Preparation and in vitro evaluation of folate-receptor-targeted SPION–polymer micelle hybrids for MRI contrast enhancement in cancer imaging. Nanotechnology. 2012;24:015603.
Vogel P, Lother S, Ruckert MA, Kullmann WH, Jakob PM, Fidler F, et al. MRI meets MPI: a bimodal MPI-MRI tomograph. IEEE Trans Med Imaging. 2014;33:1954–9.
Vaalma S, Rahmer J, Panagiotopoulos N, Duschka RL, Borgert J, Barkhausen J, et al. Magnetic particle imaging (MPI): experimental quantification of vascular stenosis using stationary stenosis phantoms. PLoS One. 2017;12:e0168902.
Sekino M, Kuwahata A, Ookubo T, Shiozawa M, Ohashi K, Kaneko M, et al. Handheld magnetic probe with permanent magnet and hall sensor for identifying sentinel lymph nodes in breast cancer patients. Sci Rep. 2018;8.
Winter A, Engels S, Reinhardt L, Wasylow C, Gerullis H, Wawroschek F. Magnetic marking and intraoperative detection of primary draining lymph nodes in high-risk prostate cancer using superparamagnetic iron oxide nanoparticles: additional diagnostic value. Molecules. 2017;22:2192.
Thill M, Kurylcio A, Welter R, van Haasteren V, Grosse B, Berclaz G, et al. The central-European SentiMag study: sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast. 2014;23:175–9.
Douek M, Klaase J, Monypenny I, Kothari A, Zechmeister K, Brown D, et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG multicentre trial. Ann Surg Oncol. 2013;21:1237–45.
Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol. 2014;15:e351–e62.
Tabatabaei S, Harisinghani M, McDougal WS. Regional lymph node staging using lymphotropic nanoparticle enhanced magnetic resonance imaging with ferumoxtran-10 in patients with penile cancer. J Urol. 2005;174:923–7.
Mirković M, Radović M, Stanković D, Milanović Z, Janković D, Matović M, et al. 99mTc–bisphosphonate–coated magnetic nanoparticles as potential theranostic nanoagent. Mater Sci Eng C. 2019;102:124–33.
Lee CM, Jeong HJ, Cheong SJ, Kim EM, Kim DW, Lim ST, et al. Prostate cancer-targeted imaging using magnetofluorescent polymeric nanoparticles functionalized with bombesin. Pharm Res. 2010;27:712–21.
Hajiramezanali M, Atyabi F, Mosayebnia M, Akhlaghi M, Geramifar P, Jalilian AR, et al. (68)Ga-radiolabeled bombesin-conjugated to trimethyl chitosan-coated superparamagnetic nanoparticles for molecular imaging: preparation, characterization and biological evaluation. Int J Nanomedicine. 2019;14:2591–605.
Riberdy V, Litvack M, Stirrat E, Couch M, Post M, Santyr GE. Hyperpolarized (129) Xe imaging of embryonic stem cell-derived alveolar-like macrophages in rat lungs: proof-of-concept study using superparamagnetic iron oxide nanoparticles. Magn Reson Med 2019.
Liu X, Du C, Li H, Jiang T, Luo Z, Pang Z, et al. Engineered superparamagnetic iron oxide nanoparticles (SPIONs) for dual-modality imaging of intracranial glioblastoma via EGFRvIII targeting. Beilstein J Nanotechnol. 2019;10:1860–72.
Salehnia Z, Shahbazi-Gahrouei D, Akbarzadeh A, Baradaran B, Farajnia S, Naghibi M. Synthesis and characterisation of iron oxide nanoparticles conjugated with epidermal growth factor receptor (EGFR) monoclonal antibody as MRI contrast agent for cancer detection. IET Nanobiotechnol. 2019;13:400–6.
Ordóñez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–28.
Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, et al. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–9.
Akaogi K, Okabe Y, Sato J, Nagashima Y, Yasumitsu H, Sugahara K, et al. Specific accumulation of tumor-derived adhesion factor in tumor blood vessels and in capillary tube-like structures of cultured vascular endothelial cells. Proc Natl Acad Sci. 1996;93:8384–9.
Croix BS. Genes expressed in human tumor endothelium. Science. 2000;289:1197–202.
Pen A, Moreno MJ, Martin J, Stanimirovic DB. Molecular markers of extracellular matrix remodeling in glioblastoma vessels: microarray study of laser-captured glioblastoma vessels. Glia. 2007;55:559–72.
Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, Chen X, PET of vascular endothelial growth factor receptor expression. J Nucl Med 2006; 47 (12): 2048-56.
Kaul MG, Mummert T, Jung C, Salamon J, Khandhar AP, Ferguson RM, et al. In vitro and in vivo comparison of a tailored magnetic particle imaging blood pool tracer with Resovist. Phys Med Biol. 2017;62:3454–69.
Polito C, Pani R, Frantellizzi V, De Vincentis G, Pellegrini R. Imaging performances of a small FoV gamma camera based on CRY018 scintillation crystal. Nuclear Instrum Methods Phys Res A: Accelerators, Spectrom, Detect Assoc Equip. 2018;912:33–5.
Spindel ER, Bombesin Peptides. Handbook of Biologically Active Peptides. 2013;326–30.
Cinti MN, Scafe R, Bennati P, Lo Meo S, Frantellizzi V, Pellegrini R, et al. Innovative LuYAP:Ce array for PET imaging. J Instrum. 2017;12.
Polito C, Pani R, Trigila C, Cinti MN, Fabbri A, Frantellizzi V, et al. Imaging characterization of a new gamma ray detector based on CRY019 scintillation crystal for PET and SPECT applications. J Instrum. 2017;12.
Ebrahimi Fard A, Zarepour A, Zarrabi A, Shanei A, Salehi H. Synergistic effect of the combination of triethylene-glycol modified Fe 3 O 4 nanoparticles and ultrasound wave on MCF-7 cells. J Magn Magn Mater. 2015;394:44–9.
Mahmoudi M, Simchi A, Vali H, Imani M, Shokrgozar MA, Azadmanesh K, et al. Cytotoxicity and cell cycle effects of bare and poly(vinyl alcohol)-coated Iron oxide nanoparticles in mouse fibroblasts. Adv Eng Mater. 2009;11:B243–B50.
Mahmoudi M, Simchi A, Imani M, Shokrgozar MA, Milani AS, Hafeli UO, et al. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf B: Biointerfaces. 2010;75:300–9.
Jeng HA, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41:2699–711.
Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol in Vitro. 2005;19:975–83.
Ankamwar B, Lai TC, Huang JH, Liu RS, Hsiao M, Chen CH, et al. Biocompatibility of Fe(3)O(4) nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology. 2010;21:75102.
Naqvi S, Samim M, Abdin M, Ahmed FJ, Maitra A, Prashant C, et al. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomedicine. 2010;5:983–9.
Xin-Li L, Shu-Hua Z, Long Z, Gui-Qin H, Sun ZW, Yang W. Dose-dependent Cytotoxicity and Oxidative Stress Induced by "Naked" Fe 3 O 4 Nanoparticles in Human Hepatocyte. 2012; 28.
Yarjanli Z, Ghaedi K, Esmaeili A, Rahgozar S, Zarrabi A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017;18:51.
Imam SZ, Lantz-McPeak SM, Cuevas E, Rosas-Hernandez H, Liachenko S, Zhang Y, et al. Iron oxide nanoparticles induce dopaminergic damage: in vitro pathways and in vivo imaging reveals mechanism of neuronal damage. Mol Neurobiol. 2015;52:913–26.
Pongrac IM, Pavicic I, Milic M, Brkic Ahmed L, Babic M, Horak D, et al. Oxidative stress response in neural stem cells exposed to different superparamagnetic iron oxide nanoparticles. Int J Nanomedicine. 2016;11:1701–15.
Riasat R, Nie G. Synthesis and characterization of nontoxic hollow Iron oxide (α-Fe2O3) nanoparticles using a simple hydrothermal strategy. J Nanomater. 2016;2016:1–7.
Sonmez E, Aydin E, Turkez H, Özbek E, Togar B, Meral K, et al. Cytotoxicity and genotoxicity of iron oxide nanoparticles: an in vitro biosafety study. Arch Biol Sci. 2016;68:41–50.
Evans SJ, Clift MJD, Singh N, Wills JW, Hondow N, Wilkinson TS, et al. In vitro detection of in vitro secondary mechanisms of genotoxicity induced by engineered nanomaterials. Part Fibre Toxicol. 2019;16:8.
Gualdani R, Guerrini A, Fantechi E, Tadini-Buoninsegni F, Moncelli MR, Sangregorio C. Superparamagnetic iron oxide nanoparticles (SPIONs) modulate hERG ion channel activity. Nanotoxicology 2019; 1-13.
Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–7.
Veranth JM, Kaser EG, Veranth MM, Koch M, Yost GS. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part Fibre Toxicol. 2007;4:2.
Hafeli UO, Riffle JS, Harris-Shekhawat L, Carmichael-Baranauskas A, Mark F, Dailey JP, et al. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol Pharm. 2009;6:1417–28.
Singh N. Conference scene - nanotoxicology: health and environmental impacts. Nanomedicine (London). 2009;4:385–90.
Wang H, Kumar R, Nagesha D, Duclos RI Jr, Sridhar S, Gatley SJ. Integrity of (111)in-radiolabeled superparamagnetic iron oxide nanoparticles in the mouse. Nucl Med Biol. 2015;42:65–70.
Jung CW. Surface properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging. 1995;13:675–91.
McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliv Rev. 2008;60:1241–51.
Wang S, Zhang B, Su L, Nie W, Han D, Han G, et al. Subcellular distributions of iron oxide nanoparticles in rat brains affected by different surface modifications. J Biomed Mater Res A. 2019;107:1988–98.
Anzai Y, Piccoli CW, Outwater EK, Stanford W, Bluemke DA, Nurenberg P, et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology. 2003;228:777–88.
Ros PR, Freeny PC, Harms SE, Seltzer SE, Davis PL, Chan TW, et al. Hepatic MR imaging with ferumoxides: a multicenter clinical trial of the safety and efficacy in the detection of focal hepatic lesions. Radiology. 1995;196:481–8.
Fulop T, Nemes R, Meszaros T, Urbanics R, Kok RJ, Jackman JA, et al. Complement activation in vitro and reactogenicity of low-molecular weight dextran-coated SPIONs in the pig CARPA model: correlation with physicochemical features and clinical information. J Control Release. 2018;270:268–74.
Birkhauser FD, Studer UE, Froehlich JM, Triantafyllou M, Bains LJ, Petralia G, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol. 2013;64:953–60.
Triantafyllou M, Studer UE, Birkhauser FD, Fleischmann A, Bains LJ, Petralia G, et al. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur J Cancer. 2013;49:616–24.
Perez-Gil J, Weaver TE. Pulmonary surfactant pathophysiology: current models and open questions. Physiology (Bethesda). 2010;25:132–41.
Kononenko V, Erman A, Petan T, Krizaj I, Kralj S, Makovec D, et al. Harmful at non-cytotoxic concentrations: SiO2-SPIONs affect surfactant metabolism and lamellar body biogenesis in A549 human alveolar epithelial cells. Nanotoxicology. 2017;11:419–29.
Moret F, Selvestrel F, Lubian E, Mognato M, Celotti L, Mancin F, et al. PEGylation of ORMOSIL nanoparticles differently modulates the in vitro toxicity toward human lung cells. Arch Toxicol. 2015;89:607–20.
Al Faraj A, Shaik AP, Shaik AS. Effect of surface coating on the biocompatibility and in vivo MRI detection of iron oxide nanoparticles after intrapulmonary administration. Nanotoxicology. 2015;9:825–34.
Mahmoudi M, Laurent S, Shokrgozar MA, Hosseinkhani M. Toxicity evaluations of superparamagnetic iron oxide nanoparticles: cell "vision" versus physicochemical properties of nanoparticles. ACS Nano. 2011;5:7263–76.
Laurent S, Saei AA, Behzadi S, Panahifar A, Mahmoudi M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expert Opin Drug Deliv. 2014;11:1449–70.
Yang WJ, Lee JH, Hong SC, Lee J, Lee J, Han DW. Difference between toxicities of Iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials (Basel). 2013;6:4689–706.
Rivet CJ, Yuan Y, Borca-Tasciuc DA, Gilbert RJ. Altering iron oxide nanoparticle surface properties induce cortical neuron cytotoxicity. Chem Res Toxicol. 2012;25:153–61.
Hoh CK, Wallace AM, Vera DR. Preclinical studies of [(99m)Tc]DTPA-mannosyl-dextran. Nucl Med Biol. 2003;30:457–64.
Aliakbari M, Mohammadian E, Esmaeili A, Pahlevanneshan Z. Differential effect of polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles on BT-474 human breast cancer cell viability. Toxicol in Vitro. 2019;54:114–22.
Maurizi L, Papa AL, Dumont L, Bouyer F, Walker P, Vandroux D, et al. Influence of surface charge and polymer coating on internalization and biodistribution of polyethylene glycol-modified Iron oxide nanoparticles. J Biomed Nanotechnol. 2015;11:126–36.
Buscombe J, Paganelli G, Burak ZE, Waddington W, Maublant J, Prats E, et al. Sentinel node in breast cancer procedural guidelines. Eur J Nucl Med Mol Imaging. 2007;34:2154–9.
Khera SY, Kiluk JV, Hasson DM, Meade TL, Meyers MP, Dupont EL, et al. Pregnancy-associated breast cancer patients can safely undergo lymphatic mapping. Breast J. 2008;14:250–4.
Pandit-Taskar N, Dauer LT, Montgomery L, St Germain J, Zanzonico PB, Divgi CR. Organ and fetal absorbed dose estimates from 99mTc-sulfur colloid lymphoscintigraphy and sentinel node localization in breast cancer patients. J Nucl Med. 2006;47:1202–8.
Gentilini O, Cremonesi M, Toesca A, Colombo N, Peccatori F, Sironi R, et al. Sentinel lymph node biopsy in pregnant patients with breast cancer. Eur J Nucl Med Mol Imaging. 2010;37:78–83.
Stratmann SL, McCarty TM, Kuhn JA. Radiation safety with breast sentinel node biopsy. Am J Surg. 1999;178:454–7.
Brenner W, Ostertag H, Peppert E, Czech N, Kampen WU, Muhle C, et al. Radiation exposure to the personnel in the operating room and in the pathology due to SLN detection with Tc-99m-nanocolloid in breast cancer patients. Nuklearmedizin. 2000;39:142–5.
Klausen TL, Chakera AH, Friis E, Rank F, Hesse B, Holm S. Radiation doses to staff involved in sentinel node operations for breast cancer. Clin Physiol Funct Imaging. 2005;25:196–202.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Viviana Frantellizzi, Miriam Conte, Mariano Pontico, Arianna Pani, Roberto Pani, and Giuseppe De Vincentis declare that they have no conflict of interest.
Ethical Approval
This work does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frantellizzi, V., Conte, M., Pontico, M. et al. New Frontiers in Molecular Imaging with Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Efficacy, Toxicity, and Future Applications. Nucl Med Mol Imaging 54, 65–80 (2020). https://doi.org/10.1007/s13139-020-00635-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-020-00635-w